Cuscuta australis (PROTA)
Introduction |
Cuscuta australis R.Br.
- Protologue: Prodr. 491 (1810).
- Family: Convolvulaceae
- Chromosome number: 2n = 28, 56
Synonyms
- Grammica australis (R.Br.) Hadac & Chrtek (1970).
Vernacular names
- Australian dodder, southern dodder (En).
- Cuscute du sud, cuscute australe (Fr).
- Enleios, cipo chumbo (Po).
- Mlangamia (Sw).
Origin and geographic distribution
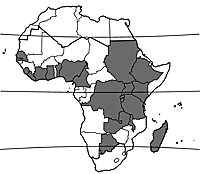
Cuscuta australis occurs in large parts of tropical and subtropical Africa, as well as in southern Europe, Asia and Australia. In tropical Africa it is recorded from Senegal east to Ethiopia and southward to South Africa. It also occurs in Madagascar.
Uses
While medicinal use of Cuscuta australis is of great importance in Asia, its use in Africa has been reported only occasionally. In Nigeria a decoction of the whole plant is used as a laxative, anthelmintic, astringent and for the treatment of sores, measles, kidney and liver diseases. In South-East Asia a plant extract is used as emollient, sedative, sudorific and tonic, and to treat urinary complaints. In Chinese traditional medicine, the seeds, together with those of Cuscuta chinensis Lam. and sometimes Cuscuta japonica Choisy, named ‘tu si zi’ or ‘semen cuscutae’, are credited with a neutral nature and a pungent, sweet taste. They are associated with the liver and kidneys and are used in formulas that help both yin and yang deficiencies, depending on the patient’s condition and the other herbs in the formula. The seeds are applied for a large number of complaints. They are used as a sedative, against diabetes, urinary complaints, impotence and sterility, deficient activity of liver, kidneys and spleen, and opacity of the cornea; they are often used in combination with other plant products.
Because of the tangled manner of growth, the plant is used in West Africa as a love-charm or to test affection. The Yoruba in Nigeria use the plant in an invocation to conceal a secret.
Production and international trade
Seed of Cuscuta australis and also of Cuscuta chinensis is widely traded in Asia and on a smaller scale worldwide. No quantitative data on production or trade are available.
Properties
Numerous compounds, including several flavonoids, have been isolated from the seeds of Cuscuta australis particularly kaempferol and derivatives, astragalin, quercetin and hyperoside. Other compounds include thymidine, caffeic acid, caffeic-β-D-glucoside, p-coumaric acid, australiside A (a diterpenoid kaurene-6-O-glucoside), and several cuscutic acids (triglycosides with jalapinolic or convolvulinolic acid as the aglycon). Volatile terpenoids identified in significant quantities include cinnamate, methyl cinnamate, dihydro-5, 6-dehydrokavain and myristic acid.
An alcoholic extract from the stems increased phagocytosis of macrophages as well as haemolysis and proliferation of lymphocytes up to normal level in mice with second degree burn injury covering 50% body surface. It was concluded that the plant may serve as an immunopotentiator for mammals. Extracts of the seeds have shown moderate activity against Gram-positive bacteria. However, the extract had little effect on a bacterial surface protein involved in attachment to cells of mammals. In a test in Nigeria, hexane, butanol and 50% ethanol extracts of the whole plant showed little or no antibacterial activity. An ethanol extract of the seeds has shown in vitro cytotoxicity, and an aqueous extract showed reduced tyrosinase activity.
In Vietnam, plants of Cuscuta australis incorporated in the soil of paddy fields have shown weed-growth reducing activity.
Adulterations and substitutes
In the Chinese medicine ‘tu si zi’, seed of Cuscuta australis and Cuscuta chinensis is used interchangeably in spite of differences in chemical composition. The species can be distinguished by comparing the quantitative proportions of indicator chemical compounds. Seed of Cuscuta japonica is also a common ingredient of ‘tu si zi’, but is reported to add little to its pharmacological properties.
Description
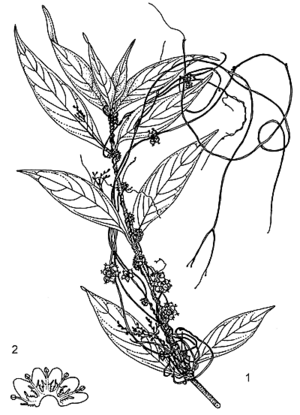
Twining, herbaceous, obligate stem-parasite, annual or rarely perennial. Roots absent except in seedling. Stems slender, up to 0.5 mm in diameter, yellowish or reddish, almost no chlorophyll, attached to host with haustoria. Leaves reduced to scales. Inflorescence a compact cymose glomerule. Flowers bisexual, regular, 4–5-merous; pedicel 1–1.5 mm long; calyx cup-shaped, as long as corolla tube, 4–5-lobed, lobes rounded, obtuse; corolla campanulate, 2–3 mm long, 4–5-lobed, lobes rounded to obtuse, white or creamy white, united into a short tube at base, a whorl of thin fringed scales near stamens; stamens 4–5, inserted at throat and alternating with corolla-lobes, up to 1 mm long; ovary superior, 2-celled, styles 2, 0.5–1 mm long, divergent. Fruit a depressed-globose capsule enclosed in persistent corolla, 3–4 mm in diameter, irregularly opening or indehiscent, reddish-brown when dry, usually 4-seeded. Seeds ellipsoid to ovoid, up to 1.5 mm long, dark reddish brown. Embryo and seedling thread-like, without cotyledons.
Other botanical information
Cuscuta is nearly cosmopolitan and comprises yellow, orange, red or rarely green parasitic plants. The highest diversity of the 100–200 species is found in the Americas. It is sometimes treated as the only genus in the family Cuscutaceae, but both morphologic and genetic research have shown that it is correctly placed in Convolvulaceae, subfamily Cuscuteae. Cuscuta campestris and the more robust Cuscuta reflexa Roxb. are the most noxious dodder weeds worldwide. In Asia Cuscuta australis is often confused with Cuscuta chinensis, which differs in its fruit, which opens by splitting around the base, and its keeled corolla lobes.
Several other Cuscuta species are used in traditional medicine in tropical Africa.
Cuscuta campestris
Cuscuta campestris Yunck (golden dodder, field dodder) originates from America and is naturalized throughout most of East and southern Africa. It further occurs in northern Africa and the Indian Ocean islands, and is widely naturalized in Asia. A decoction of crushed Cuscuta campestris stems and fresh leaves of Artemisia sp. is taken to treat abdominal cramps. The analgesic, antipyretic, anti-inflammatory and CNS-depressant activities of the dried ethanol extract of whole plants (collected from Egypt) were investigated in rats and mice. The extract protected mice against benzoquinone-induced writhing, exhibited hypothermic properties and inhibited oedema formation in carrageenan-treated rats. The CNS-depressant activity of the extract seems to be due to a tranquillizing effect.
Cuscuta reflexa
Cuscuta reflexa Roxb. (giant dodder, Indian dodder) occurs in tropical and subtropical parts of Asia and the Americas, where it can be a serious weed. It is reported to be present and used medicinally in Mauritius, where an infusion of the whole plant is used as diuretic and against gout. In Asia, the plant is important in traditional medicine, and a preparation of the whole plant is used as a purgative and also against headaches, jaundice, and in wound treatment. Phytochemical compounds isolated from the stems include: swarnalin, scoparone, melanettin, cuscutin, cuscutalin, hyperoside, taxifolin, astragalin, myricetin, kaempferol, luteolin, quercetin, reflexin and several flavonoid glycosides. The methanolic stem extract showed significant antibacterial activity in vitro. The tetrahydrofuran derivative swarnalin showed significant free radical scavenging activity in vitro. An ethanolic plant extract decreased arterial blood pressure in guinea pig atria and inhibited both histamine and acetylcholine induced contractions of guinea pig ileum. Extracts showed variable effects on smooth muscles of rat, rabbit, guinea pig, frog and dog. A water extract showed anti-HIV activity in vitro.
Growth and development
Although Cuscuta plants are capable of limited photosynthesis, they obtain nearly all of their energy from the host plant. A seedling can survive several days without a host, but if one is not found within 5 to 10 days, the seedling will die. As plants grow, they continually reattach to the host and, when other suitable hosts are nearby, shoots spread from host plant to host plant often forming a dense mat of intertwining stems.
Cuscuta australis flowers throughout the rainy season, but seed set is highest at the end of the rainy season. Seed production generally begins near the initial attachment and proceeds outward from this point; each plant is capable of producing 1000–2000 seeds.
Ecology
Cuscuta australis occurs as a parasite on numerous dicotyledonous, herbaceous or shrubby plants. It has a preference for wetter localities, e.g. along watercourses, and occurs from sea-level up to 2000 m altitude.
Propagation and planting
Seed germinates at or very near the soil surface starting at the beginning of the growing season. Moist soil and sunlight is required for germination. Germination occurs independently of host plant influence. Cuscuta seed may remain viable in the soil for many years, up to 50 years has been recorded. Generally only about 5% of the seed germinates during the year following its production.
The germinating seed sends up a yellowish, threadlike, leafless stem twining counter-clockwise and coiling around any object, including host plants. It is dependent on carbohydrates stored in the seed until it attaches to a suitable host. When the seedling contacts a host, the stem coils a few turns tightly around it and produces haustoria on the stem that penetrate the host’s vascular tissue, both phloem and xylem. Cutin is exuded by the haustorium initial that bonds it to its host. As soon as the plant starts extracting nutrients and water from the host its connection to the soil dries.
The seed lacks obvious dispersal mechanisms and is likely spread by man through the movement of soil, equipment or contaminated crop seeds. Water movement may play a role in seed dispersal as plants often grow near streams. Dormancy is due to a water-impermeable seed coat and may be broken by ambient temperatures of 35/20°C or mechanical scarification.
Management
In tests in China, Glycine max (L.) Merr., Scutellaria baicalensis Georgi, Lespedeza davurica (Laxm.) Schindl. and Vigna radiata (L.) R.Wilczek have been shown to be good hosts for Cuscuta australis.
To control Cuscuta infestation in crops, prevention by the use of clean seed and destroying sources of infestation on wild vegetation continue to be of primary importance, while mechanical removal by hand to prevent seeding remains the main option for the small farmer. Separating the seeds of parasite and crops such as nigerseed (Guizotia abyssinica (L.f.) Cass.) using special equipment is difficult and expensive. Dry heat treatment (100°C for 15 minutes) devitalizes the seed of Cuscuta species in nigerseed to be used as bird-seed, but it is not known if this method is suitable for seed to be used for sowing. The options for herbicide use have improved slightly with the development of glyphosate and more intensive evaluation of pre- and postemergence herbicides in a number of other crops. Relatively little work has been done to select resistant crop varieties. The potential for biological control would seem to deserve more determined exploitation.
In a test in China, Cuscuta australis has been sown to control invasive weeds such as Alternanthera philoxeroides Griseb., Ipomoea cairica Sweet and Mikania micrantha Kunth. The weeds proved to be much more sensitive to Cuscuta australis than the native vegetation. The treatment reduced the presence of the weeds, opened the vegetation and contributed to increased species diversity. Long-term effects were not reported.
Diseases and pests
Several disease organisms, including phytoplasma species causing yellows disease, are known to infect Cuscuta species, but no specific information on Cuscuta australis is available. Cuscuta species are commonly damaged by insects belonging to the genera Smicronyx (Curculionidae), Melanagromyza (Agromyzidae) and Herpystis (Tortricidae). The potential for some of these for biological control is being studied in Pakistan.
Genetic resources
Cuscuta australis is widespread and common and not in danger of genetic erosion.
Prospects
The taxonomy of Cuscuta remains confused although progress has been made recently. However, the distribution of several species in Africa remains uncertain, as is the identity of numerous herbarium specimens. Cuscuta australis is likely to remain important in medicine, especially in China. Its use in African traditional medicine will probably remain limited. Research is needed to explore its active components and their mode of action in the various diseases against which it is used. Cuscuta australis has occasionally been reported as a weed of crops. Care is needed to prevent it from becoming an established weed. Many countries have laws that require imported crop seeds to be free of Cuscuta seed contamination.
Major references
- Deroin, T., 2001. Convolvulaceae. Flore de Madagascar et des Comores, familles 133 bis et 171. Muséum National d’Histoire Naturelle, Paris, France. pp. 11–287.
- Djadja Siti Hazar Hoesen, 2003. Cuscuta australis R.Br. In: Lemmens, R.H.M.J. & Bunyapraphatsara, N. (Editors). Plant Resources of South-East Asia No 12(3). Medicinal and poisonous plants 3. Backhuys Publishers, Leiden, Netherlands. pp. 144–145.
- Du, X.M., Sun, N.Y., Nishi, M., Kawasaki, T., Guo, Y.T. & Miyahara, K., 1999. Components of the ether-insoluble resin glycoside fraction from the seed of Cuscuta australis. Journal of Natural Products 62(5): 722–725.
- Gonçalves, M.L., 1987. Convolvulaceae. In: Launert, E. (Editor). Flora Zambesiaca. Volume 8, part 1. Flora Zambesiaca Managing Committee, London, United Kingdom. pp. 9–129.
- Li, G. & Chen, Y., 1997. Chemical constituents of Cuscuta australis R. Br. Zhongguo Zhong Yao Za Zhi 22(9): 548–550, 576.
- Parker, C. & Riches, C.R., 1993. Parasitic weeds of the world: biology and control. CAB International, Wallingford, United Kingdom. 332 pp.
- Stefanovic, S., Kuzmina, M. & Costea, M., 2007. Delimitation of major lineages within Cuscuta subgenus Grammica (Convolvulaceae) using plastid and nuclear DNA sequences. American Journal of Botany 94(4): 568–589.
- Verdcourt, B., 1963. Convolvulaceae. In: Hubbard, C.E. & Milne-Redhead, E. (Editors). Flora of Tropical East Africa. Crown Agents for Oversea Governments and Administrations, London, United Kingdom. 161 pp.
- Yuncker, T.G., 1932. The genus Cuscuta. Memoirs of the Torrey Botanical Club 18(2): 126–127.
- Xiao, J., Cui, F., Ning, T. & Zhao, W, 1990. Effects of alcohol extract from Polygonatum odoratum (Mill.) Druce and Cuscuta australis R. Br. on immunological function of mice injured by burns. Zhongguo Zhong Yao Za Zhi 15(9): 557–559, 578.
Other references
- Agha, A.M., Sattar, E.A. & Ahmed Galal, 1996. Pharmacological study of Cuscuta campestris Yuncker. Phytotherapy Research 10(2): 117–120.
- Boiteau, P., Boiteau, M. & Allorge-Boiteau, L., 1999. Dictionnaire des noms malgaches de végétaux. 4 Volumes + Index des noms scientifiques avec leurs équivalents malgaches. Editions Alzieu, Grenoble, France.
- Burkill, H.M., 1985. The useful plants of West Tropical Africa. 2nd Edition. Volume 1, Families A–D. Royal Botanic Gardens, Kew, Richmond, United Kingdom. 960 pp.
- Chang, S.-J. & Suk, K.-D., 2006. Inhibitory effects on melanin biosynthesis and tyrosinase activity, cytotoxicity in clone M-3 and antioxidant activity by Cuscuta japonica, C. australis, and C. chinensis extracts. Yakhak Hoechi 50(6): 421–428.
- Costea, M. & Tardif, F.J., 2006. The biology of Canadian weeds. 133. Cuscuta campestris Yuncker, C. gronovii Willd. ex Schult., C. umbrosa Beyr. ex Hook., C. epithymum (L.) L. and C. epilinum Weihe. Canadian Journal of Plant Science 86(1): 293–316.
- Gupta, A.K., Tandon, N. & Sharma, M. (Editors), 2008. Quality standards of Indian medicinal plants. Volume 5. Medicinal Plants Unit, Indian Council of Medical Research, New Delhi, India. 357 pp.
- Gurib-Fakim, A., Sewraj, M., Guého, J. & Dulloo, E., 1993. Medical ethnobotany of some weeds of Mauritius and Rodrigues. Journal of Ethnopharmacology 39(3): 177–185.
- He, X.H., Yang, W.Z., Ye, M., Wang,, Q. & Guo, D., 2011. Differentiation of Cuscuta chinensis and Cuscuta australis by HPLC-DAD-MS analysis and HPLC-UV quantitation. Planta Medica 77: 1950–1957.
- Khanh, T.D., Cong, L.C., Xuan, T.D., Lee, S.J., Kong, D.S. & Chung, I.M., 2008. Weed-suppressing potential of dodder (Cuscuta hygrophilae) and its phytotoxic constituents. Weed Science 56(1): 119–127.
- Lin, H.B., Lin, J.Q., Gu, H.X., Li, Y. & Lin, J.Q., 2006. Interrelation among variety, host and content of total flavonoids in dodders. Shanghai Zhongyiyao Daxue Xuebao 20(3): 66–68.
- Liu, Z.Q., Fer, A. & Lecocq, F.M., 1999. Imazaquin, a promising herbicide for control of dodder (Cuscuta spp.) in soybean (Glycine max). Weed Research 31(1): 33–40.
- Meeuse, A.D.J. & Welman, W.G., 2000. Convolvulaceae. In: Germishuizen, G. (Editor). Flora of southern Africa. Volume 28, part 1. National Botanical Institute, Pretoria, South Africa. 138 pp.
- Okiei, W., Ogunlesi, M. & Ademoye, M.A., 2009. An assessment of the antimicrobial properties of extracts of various polarities from Chasmanthera dependens, Emilia coccinea and Cuscuta australis, herbal medications for eye diseases. Journal of Applied Sciences 9(22): 4076–4080.
- Pal, D.K., Mandal, M., Senthilkumar, G.P. & Padhian, A., 2006. Antibacterial activity of Cuscuta reflexa stem and Corchorus olitorius seed. Fitoterapia 77(7–8): 589–591.
- Parker, C., 1999. Protection of crops against parasitic weeds. Crop Protection 10: 7–22.
- Schimming, T., Jenett-Siems, K., Mann, P., Tofern-Reblin, B., Milson, J., Johnson, R.W., Deroin, T., Austin, D.F. & Eich, E., 2006. Calystegines as chemotaxonomic markers in the Convolvulaceae. Phytochemistry 66(4): 469–480.
- Uddin, S.J., Shilpi, J.A., Middleton, M., Byres, M., Shoeb, M., Nahar, L. & Sarker, S.D., 2007. Swarnalin and cis -swarnalin, two new tetrahydrofuran derivatives with free radical scavenging activity, from the aerial parts of Cuscuta reflexa. Natural Product Research 21(7): 663–668.
- Ye, M., Li, Y., Yan, Y.M., Liu, H.W. & Ji, X.H., 2002. Determination of flavonoids in Semen Cuscutae by RP-HPLC. Journal of Pharmaceutical and Biomedical Analysis 28: 621–628.
- Yu, H., Liu, J., He, W.-M., Miao, S.-L. & Dong, M., 2011. Cuscuta australis restrains three exotic invasive plants and benefits native species. Biological Invasions 13: 747–756.
Afriref references
Sources of illustration
- van Oostroom, S.J. & Hoogland, R.D., 1951. Convolvulaceae In: van Steenis, C.G.G.J. (Editor): Flora Malesiana. Series 1, Volume 4. Noordhoff-Kolff, Djakarta, Indonesia. pp. 388–512.
Author(s)
- N. Nyunaï, Institut de Recherches Médicales et d’Etudes des Plantes Médicinales, B.P. 3805, Yaoundé, Cameroon
Correct citation of this article
Nyunaï, N., 2013. Cuscuta australis R.Br. In: Schmelzer, G.H. & Gurib-Fakim, A. (Editors). Prota 11(2): Medicinal plants/Plantes médicinales 2. PROTA, Wageningen, Netherlands. Accessed 18 December 2024.
- See this page on the Prota4U database.