Cryptolepis sanguinolenta (PROTA)
Introduction |
| General importance | |
| Geographic coverage Africa | |
| Geographic coverage World | |
| Vegetable | |
| Dye / tannin | |
| Medicinal | |
| Fibre | |
| Food security | |
Cryptolepis sanguinolenta (Lindl.) Schltr.
- Protologue: Westafr. Kautschuk-Exped. 308 (1900).
- Family: Asclepiadaceae (APG: Apocynaceae)
- Chromosome number: 2n = 22
Origin and geographic distribution
Cryptolepis sanguinolenta is found from Senegal east to Nigeria, and has also been recorded from Cameroon, Central African Republic, Congo, DR Congo, Uganda, Tanzania and Angola.
Uses
The roots of Cryptolepis sanguinolenta are a source of a yellow dye used for dyeing leather, e.g. in Côte d’Ivoire and Nigeria for tanned goat skins. The roots are crushed and ground in a wooden mortar, a little hot water is added and the mixture is stirred. It has a dull yellow colour and should be used immediately. The tanned goat skin surface is first treated with some groundnut oil and subsequently dipped in the dye bath, the extract being well rubbed into the surface. After a few minutes a piece of tamarind (Tamarindus indica L.) fruit paste is added. This paste, prepared from seeds and fruit pulp, is soaked in cold water and gradually warmed up until a pulpy mass develops. This new mixture is also rubbed in. The skin is exposed to the air for 2–3 minutes and after that again rubbed in with the mixture for about 5 minutes, wiped clean and hung up to dry. The effect of the tamarind fruit paste is to purify the colour as it removes the red tint that would result from the addition of alkali in the dye bath.
In traditional medicine the bitter roots are widely used for different purposes. The roots are chewed while the root bark or root extracts are taken to treat fever, hepatitis (Guinea Bissau), malaria (Ghana), hypertension and urinary and upper respiratory tract infections (Ghana, Nigeria), colic, stomach complaints, amoebic dysentery and diarrhoea (Senegal, Ghana, Nigeria, DR Congo, Uganda), wounds, measles, hernia, snakebites (Uganda), rheumatism (Senegal, Nigeria), insomnia (Ghana) and as a tonic (Senegal, Nigeria).
In Burkina Faso the leaves are used as a vegetable. In Uganda the stem is used as a rope in house construction.
Production and international trade
The roots of Cryptolepis sanguinolenta are collected from the wild and locally sold on markets in West Africa for medicinal purposes and for dye extraction. Some antimalarial medicines have been developed such as ‘Phytolaria’ in Ghana and ‘Malarial’ in Mali. The raw powdered plant and the freeze-dried aqueous extract have also been formulated into tablets and suppositories.
Properties
The leaves and roots of Cryptolepis sanguinolenta are rich in bioactive indole alkaloids such as cryptolepine, the major constituent of the root bark and the first alkaloid isolated from the plant. From the roots a range of structurally related alkaloids has been isolated, including hydroxycryptolepine, isocryptolepine, cryptospirolepine, neocryptolepine, neocryptine and quindoline. In an aqueous extract of the roots only cryptolepine was detected, and it constitutes the main or only source of yellow colorant in the dye baths. Other yellow alkaloid dyes such as berberin from Berberis and Mahonia species have had historical importance in Asia, Europe and America.
Cryptolepine, the most extensively investigated alkaloid of the plant, has antiplasmodial, anticancer, antifungal, antibacterial, hypotensive, antipyretic, anti-inflammatory and anti-hyperglycemic activities. Its antimalarial activity has been most extensively investigated. Cryptolepine has potent in vitro antiplasmodial activity but it also has considerable cytotoxic, genotoxic, DNA intercaling and topoisomerase II inhibition properties. However, in a previous study, it was shown that the antiplasmodial action of cryptolepine is due, at least in part, to a chloroquine-like action that does not depend on intercalation into DNA. The synthesis of a series of analogues of cryptolepine was reported, and some of these were shown to have enhanced antiplasmodial activities in vitro against both chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum. Furthermore, several compounds were found to have significant antiplasmodial activity in mice infected with Plasmodium berghei, and importantly, in contrast to cryptolepine, no apparent toxicity was seen in the mice. These results indicated that cryptolepine analogues have potential as lead compounds to new antimalarial agents. Several neocryptolepine derivatives were evaluated for their anti-malarial activity against chloroquine-sensitive as well as chloroquine-resistant Plasmodium falciparum strains. The activity against Trypanosoma brucei and Trypanosoma cruzi, as well as their cytotoxicity on human cells was evaluated. These molecules showed higher antiplasmodial activity and lower cytotoxicity than the original compound. In another test, cryptolepine and 8 synthetic analogues were assessed for in vitro activities against Trypanosoma brucei, and 4 compounds were found to be highly potent. When tested in rats, the most effective compound was found to be 2, 7-dibromocryptolepine.
Cryptolepine hydrochloride showed significant activity against the fast growing Mycobacterium fortuitum, which has recently been shown to be of use in the evaluation of anti-tubercular drugs. Activity was also observed against 5 other Mycobacterium spp. Several analogues of cryptolepine showed increased potency compared to cryptolepine against Candida albicans, Cryptococcus neoformans and Aspergillus niger. Comparison of several of these analogues with standard antifungal agents showed that they were as potent as regular antifungal medicines. An ethanolic root bark extract showed potent anti-bacterial and moderate antiviral activities. The isolated compounds cryptolepine, hydroxycryptolepine, neocryptolepine, biscryptolepine and quindoline strongly inhibited the growth of Gram-positive bacteria and showed moderate or no activity against selected Gram-negative bacteria. They also possessed a bactericidal effect depending on the bacterial strain. Cryptolepine was found to possess moderate activity against Herpes simplex virus. The antibacterial activity of neocryptolepine and biscryptolepine was bacteriostatic rather than bactericidal. No antifungal activity was observed for the alkaloids against Epidermophyton floccosum, Trichophyton rubrum and Aspergillus fumigatus. The minimal inhibitory concentrations of cryptolepine, as well as ethanol and aqueous root extracts were determined for 65 strains of Campylobacter jejuni, 41 strains of Campylobacter coli and 86 strains of Vibrio cholerae. The ethanol extract activity against Campylobacter strains is higher than that of commercial drugs. The ethanol extract and cryptolepine showed some activity against the Vibrio cholerae strains.
Cryptolepine significantly lowers glucose when given orally to a mouse model of diabetes type 2. The antihyperglycaemic effect of cryptolepine leads to a significant decline in plasma insulin concentration, associated with evidence of an enhancement in insulin-mediated glucose disposal.
Cryptolepine reduced the contraction of isolated guinea-pig ileum induced by acetylcholine or prostaglandin E2. It also reduced the twitch response of the ileum evoked by coaxial electrical stimulation. Further studies confirmed the anticholinergic activities of cryptolepine.
Furthermore, an aqueous root extract showed anxiogenic but also anxiolytic effects in different tests with mice. The extract reduced spontaneous locomotor activity in mice and prolongs pentobarbitone sleeping time but induced anxiogenic-like behavior in another study. It is plausible that the sedative and anxiogenic-like effects observed may be by different mechanisms on different receptors.
Hydroxycryptolepine has been shown to inhibit xanthine oxidase and acts as a scavenger of superoxide anions.
A study on the impact of environmental factors on the levels of total alkaloid content of different plant parts of Cryptolepis sanguinolenta from different locations in Ghana showed that fluctuations in temperature, rainfall, relative humidity and duration of sunshine influenced the alkaloid content in different parts of the plants.
Description
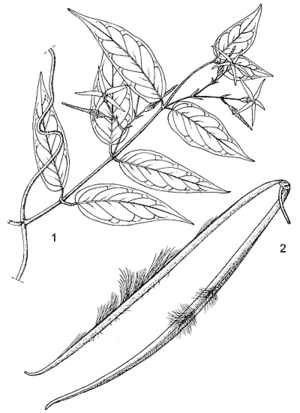
- Twining and scrambling shrub with thin branches up to 8 m long, containing yellow-orange juice becoming red upon drying.
- Leaves opposite, simple and entire, glabrous; stipules absent; petiole 1–1.5 cm long; blade elliptical to ovate, 2.5–10 cm × 1–6 cm, base rounded to cuneate, apex acuminate.
- Inflorescence an axillary cyme up to 8 cm long, with few, laxly spaced flowers.
- Flowers bisexual, regular, 5-merous, c. 1.5 cm long, greenish yellow; pedicel 0.5–1.5 cm long; sepals lanceolate, acute; corolla with tube c. 5 mm long, lobes arranged star-like, lanceolate, c. 12 mm long, contorted to the left in bud.
- Fruit a pair of spreading follicles, each narrowly cylindrical and up to 18 cm × 0.5 cm.
- Seeds c. 12 mm long, bearing a coma of long silky hairs.
Other botanical information
Cryptolepis belongs to subfamily Periplocoideae. It comprises about 30 species in Africa, Asia and Australia. The majority of the species occur in East Africa and on Socotra (Yemen). The distribution and chemical composition of Cryptolepis sanguinolenta is quite different from the other African Cryptolepis species. The leaves of most Cryptolepis species contain phenolic compounds, but these are absent in the leaves of Cryptolepis sanguinolenta. However, the leaves of Cryptolepis sanguinolenta contain alkaloids, which are rare in other Cryptolepis species.
Cryptolepis eburnea
Another Cryptolepis species occurring in the region is used medicinally. Cryptolepis eburnea (Pichon) Venter occurs in the forest area of West Africa. In Sierra Leone the bark latex is applied to the skin to treat craw-craw (onchocerciasis).
Ecology
Cryptolepis sanguinolenta occurs in savanna, dry forest and in gallery forest, usually near water, from sea-level up to 850 m altitude.
Propagation and planting
The seeds of Cryptolepis sanguinolenta lose viability quickly; however, when freshly harvested, the seeds showed 90–100% germination. Seedlings can be raised in polythene bags and transplanted at the start of the rainy season. Plants flower 10 months after sowing.
The viability of the seeds was tested under 3 different storage conditions: room temperature (25–28ºC), refrigerator (10–12ºC) and deep-freezer (-18– -21ºC), using a paper box, glass jar, plain polythene bag and black polythene bag as storage containers. The results indicated that none of the containers was able to prevent the seeds from declining in viability, but the plain polythene bag gave the best results. During the first 4 weeks, the viability of the seeds was best under room temperature, and at 12 weeks the viability was best in the refrigerator.
Genetic resources
Cryptolepis sanguinolenta is widespread and does not seem to be in danger of genetic erosion. In Ghana in-vitro propagation of plantlets and slow-growth storage was tested successfully as a means of conservation of genetic diversity.
Prospects
Cryptolepis sanguinolenta has received much attention the past years, mainly because of the interesting antiplasmodial and anticancer activities of cryptolepine, the most important alkaloid isolated mainly from the roots, as well as the even higher activities of a range of cryptolepine analogues with lower cytotoxicity. Root extracts and different isolated alkaloids also showed significant activities against a range of bacteria and fungi, thus confirming its use in traditional medicine. More research is however needed to evaluate the potential of the alkaloids and to assess the safety of use in local medicine. Cryptolepis sanguinolenta will remain locally important as a source of yellow dye.
Major references
- Ameyaw, Y., Akotoye, H.K. & Boahen, Y.O., 2007. The effect of environmental factors on the total alkaloid contents of Cryptolepis sanguinolenta (Lindl.) Schtr. International Journal of Chemical Sciences 5(2): 462–476.
- Ansah, C., Mfoafo, E.A., Woode, E., Opoku-Okrah, C., Owiredu, W.K.B.A. & Duwiejua, M., 2008. Toxicological evaluation of the anti-malarial herb Cryptolepis sanguinolenta in rodents. Journal of Pharmacology and Toxicology 3(5): 335–343.
- Berhaut, J., 1971. Flore illustrée du Sénégal. Dicotylédones. Volume 1. Acanthacées à Avicenniacées. Gouvernement du Sénégal, Ministère du Développement Rural et de l’Hydraulique, Direction des Eaux et Forêts, Dakar, Senegal. 626 pp.
- Burkill, H.M., 1997. The useful plants of West Tropical Africa. 2nd Edition. Volume 4, Families M–R. Royal Botanic Gardens, Kew, Richmond, United Kingdom. 969 pp.
- Dalziel, J.M., 1926. African leather dyes. Kew Bulletin 1926: 225–238.
- Frederich, M., Tits, M. & Angenot, L., 2008. Potential antimalarial activity of indole alkaloids. Transactions of the Royal Society of Tropical Medicine and Hygiene 102: 11–19.
- Neuwinger, H.D., 2000. African traditional medicine: a dictionary of plant use and applications. Medpharm Scientific, Stuttgart, Germany. 589 pp.
- Oluwafemi, A.J., Okanla, E.O., Camps, P., Munoz-Torrero, D., Mackey, Z.B., Chiang, P.K., Seville, S. & Wright, C.W., 2009. Evaluation of Cryptolepine and Huperzine derivatives as lead compounds towards new agents for the treatment of human African trypanosomiasis. Natural Product Communications 4(2):193–198
- van Miert, S., Hostyn, S., Maes, B. U W, Cimanga, K., Brun, R., Kaiser, M., Matyus, P., Dommisse, R., Lemiere, G., Vlietinck, A. & Pieters, L., 2005. Isoneocryptolepine, a synthetic indoloquinoline alkaloid, as an antiplasmodial lead compound. Journal of Natural Products 68(5): 674–677.
- Wright, C.W., 2007. Recent developments in naturally derived antimalarials: cryptolepine analogues. Journal of Pharmacy and Pharmacology 59(6): 899–904.
Other references
- Ablordeppey, S.Y., Fan, P., Li, S., Clark, A.M. &. Hufford, C.D., 2002. Substituted indoquinolines as new antifungal agents. Bioorganic & Medicinal Chemistry 10: 1337–1346.
- Adjebeng-Danquah, J., 2003. Viability studies in Cryptolepis sanguinolenta. B.Sc. Agriculture degree thesis, Department of Crop Science, Faculty of Agriculture, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. 53 pp.
- Amoasi, M.O., 2007. Formulation and in vitro evaluation of crude extract of Cryptolepis sanguinolenta tablets. B.Pharm. Degree Thesis, Faculty of Pharmacy and Pharmaceutical Sciences, College of Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. 29 pp.
- Ansah, C., Mfoafo, E. A A, Woode, E. & Duwiejua, M., 2008. Anxiogenic effects of an aqueous crude extract of Cryptolepis sanguinolenta (Periplocaceae) in mice. International Journal of Pharmacology 4(1): 20–26.
- Appiah, D.A., 2006. A review on the importance of toxicity testing of herbal medicines and an investigation on the modulation of the sedative effect of diazepam by Cryptolepis. B.Pharm degree thesis, Department of Pharmacognosy, Faculty of Pharmacy and Pharmaceutical Sciences, College of Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. 33 pp.
- Bierer, D.E., Fort, D.M., Mendez, C.D., Luo-Jian, Imbach, P.A., Dubenko, L.G., Jolad, S.D., Gerber, R.E., Litvak, J., Lu-Qing, Zhang-PingSheng, Reed, M.J., Waldeck, N., Bruening, R.C., Noamesi, B.K., Hector, R.F., Carlson, T.J., King, S.R., Luo, J., Lu, Q. & Zhang, P.S., 1998. Ethnobotanical-directed discovery of the antihyperglycemic properties of cryptolepine: its isolation from Cryptolepis sanguinolenta, synthesis, and in vitro and in vivo activities. Journal of Medicinal Chemistry 41(6): 894–901.
- Ansah, C., Mfoafo, E. A A, Woode, E. & Duwiejua, M., 2008. Anxiogenic effects of an aqueous crude extract of Cryptolepis sanguinolenta (Periplocaceae) in mice. International Journal of Pharmacology 4(1): 20–26.
- Bourdy, G., Willcox, M.L., Ginsburg, H., Rasoanaivo, P., Graz, B. & Deharo, E., 2007. Ethnopharmacology and malaria: New hypothetical leads or old efficient antimalarials? International Journal for Parasitology 38(1): 33–41.
- Bullock, A.A., 1963. Periplocaceae. In: Hepper, F.N. (Editor). Flora of West Tropical Africa. Volume 2. 2nd Edition. Crown Agents for Oversea Governments and Administrations, London, United Kingdom. pp. 80–85.
- Cimanga, K., de Bruyne, T., Pieters, L., Totte, J., Tona, L., Kambu, K., vanden Berghe, D. & Vlietinck, A.J., 1998. Antibacterial and antifungal activities of neocryptolepine, biscryptolepine and cryptoquindoline, alkaloids isolated from Cryptolepis sanguinolenta. Phytomedicine 5(3): 209–214.
- Dassonneville, L., Lansiaux, A., Wattelet, A., Wattez, N., Mahieu, C., Van Miert, S., Pieters L. & Bailly, C., 2000. Cytotoxicity and cell cycle effects of the plant alkaloids cryptolepine and neocryptolepine: Relation to drug-induced apoptosis. European Journal of Pharmacology 409: 9–18.
- Jonckers, T.H., van Miert, S., Cimanga, K., Bailly, C., Colson, P., De Pauw Gillet, M.C., van den Heuvel, H., Claeys, M., Lemiere, F., Esmans, E.L., Rozenski, J., Quirijnen, L., Maes, L., Dommisse, R., Lemiere, G.L., Vlietinck, A. & Pieters, L., 2002. Synthesis, cytotoxicity, and antiplasmodial and antitrypanosomal activity of new neocryptolepine derivatives. Journal of Medicinal Chemistry 45(16): 3497–3508.
- Luo, J., Fort, D.M., Carlson, T.J., Noamesi, B.K., nii-Amon-Kotei, D., King, S.R., Tsai, J., Quan, J., Hobensack, C., Lapresca, P., Waldeck, N., Mendez, C.D., Jolad, S.D., Bierer, D.E. & Reaven, G.M., 1998. Cryptolepis sanguinolenta: an ethnobotanical approach to drug discovery and the isolation of a potentially useful new antihyperglycaemic agent. Diabetic Medicine 15(5): 367–374.
- Mertz, O., Lykke, A.M. & Reenberg, A., 2001. Importance and seasonality of vegetable consumption and marketing in Burkina Faso. Economic Botany 55: 276–289.
- Nettey, N.N., 2003. In vitro conservation of selected medicinal plants using tissue culture techniques. B.Sc. Agriculture degree thesis, Department of Crop Science, Faculty of Agriculture, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. 41 pp.
- Onyeibor, O., Croft, S.L., Dodson, H.I., Feiz-Hadda, M., Kendrick, H., Millington, N.J., Parapini, S., Phillips, R.M., Seville, S., Shnyder, S.D., Taramelli, D. & Wright, C.W., 2005. Synthesis of some Cryptolepine analogues, assessment of their antimalarial and cytotoxic activities, and consideration of their antimalarial mode of action. Journal of Medicinal Chemistry 48: 2701–2709.
- Paulo, A., Gomes, E.T., Steele, J., Warhurst, D.C. & Houghton, P.J., 2000. Antiplasmodial activity of Cryptolepis sanguinolenta alkaloids from leaves and roots. Planta Medica 66(1): 30–34.
- Paulo, A. & Houghton, P.J., 2003. Chemotaxonomic analysis of the genus Cryptolepis. Biochemical Systematics and Ecology 31: 155–166.
- Silva, O., Duarte, A., Cabrita, J., Pimentel, M., Diniz, A. & Gomes, E., 1996. Antimicrobial activity of Guinea-Bissau traditional remedies. Journal of Ethnopharmacology 50: 55–59.
- Tabuti, J.R.S., Lye, K.A. & Dhillion, S.S., 2003. Traditional herbal drugs of Bulamogi, Uganda: plants, use and administration. Journal of Ethnopharmacology 88: 19–44.
Sources of illustration
- Bullock, A.A., 1963. Periplocaceae. In: Hepper, F.N. (Editor). Flora of West Tropical Africa. Volume 2. 2nd Edition. Crown Agents for Oversea Governments and Administrations, London, United Kingdom. pp. 80–85.
Author(s)
- P.C.M. Jansen, PROTA Network Office Europe, Wageningen University, P.O. Box 341, 6700 AH Wageningen, Netherlands
- G.H. Schmelzer, PROTA Network Office Europe, Wageningen University, P.O. Box 341, 6700 AH Wageningen, Netherlands
Correct citation of this article
Jansen, P.C.M. & Schmelzer, G.H., 2010. Cryptolepis sanguinolenta (Lindl.) Schltr. In: Schmelzer, G.H. & Gurib-Fakim, A. (Editors). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. Accessed 6 March 2025.
- See the Prota4U database.
NB. This is the revised version published in Medicinals - 2. A preliminary version appeared in the volume Dyes and tannins.