Dendrocalamus giganteus (PROTA)
Introduction |
| General importance | |
| Geographic coverage Africa | |
| Geographic coverage World | |
| Vegetable | |
| Timber | |
| Ornamental | |
| Auxiliary plant | |
| Food security | |
| Climate change | |
Dendrocalamus giganteus Wall. ex Munro
- Protologue: Trans. Linn. Soc. 26(1): 150 (1868).
- Family: Poaceae (Gramineae)
- Chromosome number: 2n = 72
Vernacular names
- Giant bamboo, dragon bamboo (En).
- Bambou de Birmanie, bambou géant (Fr).
- Bambu balde, bambu imperial, bambu gigante (Po).
Origin and geographic distribution
The origin of Dendrocalamus giganteus is not known precisely, but could possibly be in southern Myanmar (Burma) and north-western Thailand. It is commonly planted in India, Sri Lanka, Bangladesh and southern China, and it has been introduced and planted in many botanical gardens. Its actual distribution in tropical Africa is unclear, but it has been recorded in Ghana, Benin, Kenya, Madagascar and Réunion.
Uses
Dendrocalamus giganteus is said to be used in Madagascar for construction, flooring, and musical instruments, but there may be confusion with the endemic Cathariostachys madagascariensis (A.Camus) S.Dransf., which is also known as ‘giant bamboo’. In Asia the large stems of Dendrocalamus giganteus are used for many purposes, e.g. construction, scaffolding and rural housing, water pipes, buckets, boat masts, matting, wicker ware and paper production. The thick-walled stems are especially suitable for the production of bamboo boards, which are an ideal material for room decoration and other interior applications such as walls, ceilings, floors, doors, shelves, etc. The young shoots are edible, but they are not widely consumed. They have a fair canning quality. In Thailand the large stem sheaths are made into hats. Dendrocalamus giganteus can be planted to protect soil against erosion. As one of the largest bamboo species, it has a high ornamental value.
Properties
At a moisture content of 19% the density of the stem walls is about 0.9 g/cm³. The modulus of rupture is 93–179 N/mm², modulus of elasticity about 14,000 N/mm², compression parallel to grain 39–62 N/mm² and shear about 4.5 N/mm². The stems are very susceptible to powder-post beetle attack.
Papermaking studies have shown that African Dendrocalamus giganteus yields pulp suitable for paper with a high tearing strength. The average dimensions of the stem fibre are: length 2.7 mm, diameter 26 μm, lumen width 19 μm, wall thickness 3.9 μm. Fibres from Dendrocalamus giganteus from Madagascar were on average 2.4 mm long with a diameter of 18 μm. The chemical composition of stems from Madagascar was: cellulose 39.4%, pentosans 18.4%, lignin 25.3%, ash 2.9%, silica 0.4%. The solubility in hot water was 5.1%, in alcohol-benzene 6.5%, in 1% NaOH 24.4%.
The shoots contain cyanogenic compounds, including taxiphyllin, and give an irritant sensation in the mouth and throat. These compounds can be removed by cooking.
Shoot residues (mainly sheaths and soft pieces of the stem) contain per 100 g dry matter: protein 13.1 g, fat 1.8 g, fibre 23.5 g, ash 6.4 g, Ca 53 mg, Mg 108 mg, P 261 mg, Fe 11 mg and Zn 5 mg. The hydrocyanic acid content is 213 mg per 100 g. The residues can be used for fodder after removal of the hydrocyanic acid by boiling.
Description
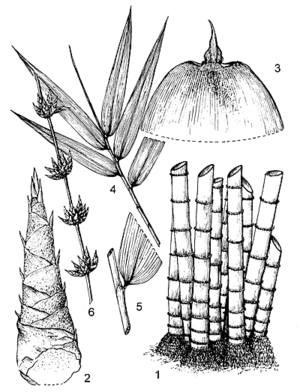
- Giant bamboo, with a short, thick rhizome and densely tufted stems; stem (culm) erect with arching tip, up to 30(–35) m tall, up to 30 cm in diameter, wall up to 25 mm thick, covered with a white waxy layer when young, becoming whitish to greyish green; internodes 25–55 cm long, lowermost ones shortest; nodes not swollen, lower ones bearing aerial roots.
- Leaves alternate, simple; stem leaves with sheath up to 50 cm × 50 cm, dark brown hairy, with small auricles, ligule up to 13 mm long and blade up to 38 cm × 9 cm; branch leaves with sheath glabrous outside, auricles small and glabrous, ligule 2–3 mm long, irregularly toothed, blade obliquely oblong, 20–50 cm × 3–10 cm, shortly stalked at base, apex acuminate, glabrous above, slightly rough, with distinct cross veins.
- Inflorescence a panicle on a leafless branch with clusters of spikelets at the nodes.
- Spikelets 12–17 mm × 3–8 mm, flattened, comprising 1–3 glumes and 4–6(–8) florets, the uppermost one sometimes sterile; glumes ovate; lemma shortly oblong, 8–13 mm long, palea of lower flowers 2-keeled and hairy at margin, that of upper flowers usually not keeled and glabrous; florets with 6 stamens having long filaments and an ovoid, hairy ovary, style long, terminating in a single feathery purple stigma.
- Fruit an oblong caryopsis (grain), 6–8 mm long, hairy above.
Other botanical information
Dendrocalamus giganteus stems grow very fast; in India average growth rates of 20 cm per day during 3.5 months have been observed. At first, the growth of a young shoot is very slow, quickening gradually during a period of 4–6 weeks, until the stem is about 4 m tall. Then the maximum growth rate is attained and maintained for several weeks. Thereafter the growth rate gradually decreases until it stops when full height is attained at the age of 3.5 months. Rapid growth seems to be induced by high relative humidity causing a high turgescence in the stem. Competition between stems in a clump may cause ‘abortive shoots’, affecting about 50% of all new shoots. Young abortion-prone shoots usually grow within 20 cm from a stem, attaining 10–15 cm in height before dying. Such young shoots are suitable for vegetable use.
Dendrocalamus giganteus flowers gregariously and the flowering cycle is estimated to be 30–40 years. It has been stated that after flowering the clump dies, but in Sri Lanka it was observed that most clumps survived after flowering. In Indonesia clumps survived when flowering stems were cut down.
Dendrocalamus comprises about 35 species, distributed from India to China and the Philippines.
Ecology
Dendrocalamus giganteus occurs naturally in humid areas at slightly higher altitudes (up to 1200 m). It can, however, be grown successfully at low altitudes on rich alluvial soils. It tolerates light frost.
Management
Propagation is normally by clump division or rhizome planting. Dendrocalamus giganteus can also be propagated by seed. Propagation by stem and branch cuttings is possible, although difficult. Rapid in-vitro multiplication is possible using nodes with axillary buds on Murashige and Skoog (MS) medium. In other studies, callus development was induced on leaves, shoots, spikelets and roots, and whole plants were obtained from callus transferred to MS medium.
In an 8 ha plantation in Myanmar, 40–50 clumps were grown per ha. Harvesting of stems may start 7 years after planting. All 3-year-old stems from mature clumps (15–16 years old) can be cut annually. A mature clump may yield 3–4 stems per year. With 50 clumps per ha, annual yield can attain up to 200 stems and 200 young shoots. The edible portion of young shoots is about 33%, or 550 g on average. The fungus Pycnoporus sanguineus and powder-post beetles may attack dry harvested stems. In Asia stems are traditionally submerged for some time in running water or in mud to obtain some protection against powder-post beetles.
Genetic resources
Germplasm collections of Dendrocalamus giganteus are maintained in Bangladesh (Forest Research Institute, Chittagong), India (Van Vigyan Kendra, Chessa, Arunachal Pradesh) and Indonesia (Lampung, Sumatra). Representative collections from all provenances are needed. In India some work is being done on the selection of superior types.
Prospects
Because of its rapid growth and long stems, Dendrocalamus giganteus may have potential in tropical Africa as an alternative for wood, e.g. in the production of board and paper and as a local source of construction material and fuel. For use as construction material, effective protection against attack by powder-post beetles is necessary.
Major references
- Clayton, W.D., Harman, K.T. & Williamson, H., 2002–. GrassBase - the online world grass flora. [Internet] Rotal Botanic Gardens, Kew, United Kingdom.http://www.kew.org/data/grasses-db/. August 2007.
- Dah-Dovonon, J., 2001. Recherches pour la promotion et le développement du bambou et du rotin dans le Sud-Bénin. In: Agbo, B.P., Arodokoun, D.Y., Aïhou, K. & Matthess, A. (Editors). Recherche agricole pour le développement. Actes de l’atelier scientifique 1, Niaouli, 11–22 janvier 2001. Programme Régional Sud-Centre du Bénin. pp. 270–285.
- Doat, J., 1967. Les bambous, source éventuelle de cellulose pour l’Afrique. Bois et Forêts des Tropiques 113: 41–59.
- Seethalakshmi, K.K. & Muktesh Kumar, M.S., 1998. Bamboos of India: a compendium. Technical Report No 17. Kerala Forest Research Institute, Peechi, Kerala, India & International Network for Bamboo and Rattan (INBAR), Beijing, China. 342 pp.
- Widjaja, E.A., 1995. Dendrocalamus giganteus Wallich ex Munro. In: Dransfield, S. & Widjaja, E.A. (Editors). Plant Resources of South-East Asia No 7. Bamboos. Backhuys Publishers, Leiden, Netherlands. pp. 85–87.
Other references
- Arya, S., Rana, P.K, Sharma, R. & Arya, I.D., 2006. Tissue culture technology for rapid multiplication of Dendrocalamus giganteus Munro. Indian Forester 132(3): 345–357.
- Azzini, A., Leme, P.R., Carvalho, C.R.L., de Barros Salgado, A.L. & Ferreira, V.L.P., 1995. Caracterização bromatológica e mineral dos resíduos de broto de bambu, visando a sua utilização como alimento animal. Bragantia 54(2): 257–261.
- Clayton, W.D., Davidse, G., Gould, F., Lazarides, M. & Soderstrom, T.R., 1994. Poaceae. In: Dassanayake, M.D. (Editor). A revised handbook to the flora of Ceylon. Vol. 8. Amerind Publishing Co., New Delhi, India. 458 pp.
- Ferreira, V.L.P., Yotsuyanagi, K. & Carvalho, C.R.L., 1995. Elimination of cyanogenic compounds from bamboo shoots Dendrocalamus giganteus Munro. Tropical Science 35(4): 342–346.
- Lin, W.C., 1978. Bambusoideae. In: Li, Hui-lin et al. (Editors): Flora of Taiwan. Vol. 5. Epoch Publishing Company, Taipei, Taiwan. pp. 706–783.
- Munoz Fonseca, M., Guevara Berger, E. & Montiel Longhi, M., 1998. Regeneración in vitro del bambú gigante Dendrocalamus giganteus (Poaceae). Revista de Biología Tropical 46: 50–56.
- Ramanayake, S.M.S.D. & Wanniarachchi, W.A.V.R., 2003. Organogenesis in callus derived from an adult giant bamboo (Dendrocalamus giganteus Wall. ex Munro). Scientia Horticulturae 98(2): 195–200.
- Ramanayake, S.M.S.D. & Yakandawala, K., 1997. Incidence of flowering, death and phenology of development in the giant bamboo (Dendrocalamus giganteus Wall. ex Munro). Annals of Botany 82(6): 779–785.
- Ramanayake, S.M.S.D. & Yakandawala, K., 1997. Micropropagation of the giant bamboo (Dendrocalamus giganteus Munro) from nodal explants of field grown culms. Plant Science 129(2): 213–223.
Sources of illustration
- Widjaja, E.A., 1995. Dendrocalamus giganteus Wallich ex Munro. In: Dransfield, S. & Widjaja, E.A. (Editors). Plant Resources of South-East Asia No 7. Bamboos. Backhuys Publishers, Leiden, Netherlands. pp. 85–87.
Author(s)
- M. Brink, PROTA Network Office Europe, Wageningen University, P.O. Box 341, 6700 AH Wageningen, Netherlands
Correct citation of this article
Brink, M., 2008. Dendrocalamus giganteus Munro. In: Louppe, D., Oteng-Amoako, A.A. & Brink, M. (Editors). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. Accessed 31 March 2025.
- See the Prota4U database.