Bridelia micrantha (PROTA)
Introduction |
Bridelia micrantha (Hochst.) Baill.
- Protologue: Adansonia 3: 164 (1862).
- Family: Euphorbiaceae (APG: Phyllanthaceae)
Synonyms
- Bridelia stenocarpa Müll.Arg. (1864).
Vernacular names
- Mitzeerie, Coast goldleaf, Yoruba ironwood, Benin ironwood (En).
- Mwiza (Sw).
Origin and geographic distribution
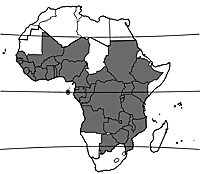
Bridelia micrantha is widely spread throughout mainland tropical Africa with the exception of a number of countries with very low annual rainfall. It has been introduced in Réunion, probably because of its medicinal properties, and has become naturalized there.
Uses
The wood is widely used for construction, poles, furniture, mortars, spoons and tool handles. It is suitable for flooring, joinery, interior trim, mine props, ship building, vehicle bodies, toys, novelties, boxes, crates, carvings, pattern making, draining boards, turnery, veneer and plywood. The wood is also used as firewood and for charcoal.
The leaves are fed to cattle. They are a favourite food of the wild silkworm (Anaphe spp.), and silk and edible caterpillars are collected from wild stands as well as from semi-domesticated plants. The fruits are sweetish and edible, and in East Africa the Maasai people use the fruits to flavour milk. In West Africa the bark is added to palm wine to improve the taste. Bridelia micrantha is planted in agroforestry systems to provide shade and mulch, and it is sometimes used for hedges. In South Africa it has been planted to restore and stabilize irrigation canals on sugarcane farms. It is planted as an ornamental and especially recommended where waterlogging is a problem. In the Sahel region, a decoction of the leaves and young branches is used as a black dye for pottery, and in Tanzania a red dye is extracted from the bark. Bark, leaves and roots have medicinal applications throughout the range of Bridelia micrantha. The bark is widely used in the treatment of wounds, and as a purgative, abortifacient and aphrodisiac, whereas in Congo bark decoctions are taken to treat cough and sore throat. In South Africa the bark is used as a remedy for headache, sore joints, sore eyes, stomach-ache, diarrhoea, tapeworms, venereal diseases and fever.The leaves are used as a laxative and chewed against headache. In Tanzania the roots are used to treat symptoms of non-insulin dependent diabetes mellitus such as excessive thirst and urine production, and sweating. In Côte d’Ivoire leaf and root extracts are applied as anthelmintic and to treat malaria and trypanosomiasis. In DR Congo the inner bark is used in the preparation of arrow poison.
Production and international trade
The timber is traded under the name ‘asas’ or ‘assas’ but quantities are small and statistics are not available. Considerable amounts of bark are traded on local markets for medicinal purposes, but also for this trade statistics are not available.
Properties
The heartwood is pale to dark brown and clearly to poorly demarcated from the greyish or yellowish white sapwood. The grain is straight to interlocked, texture fine and uneven.
The wood is medium-weight, with a density of 500–610(–705) kg/m³ at 12% moisture content. It requires careful drying to avoid distortion and checking. The rates of shrinkage are moderate, from green to oven dry 3.8–4.0% radial and 6.1–6.5% tangential. Once dry, the wood is moderately stable in service. At 12% moisture content, the modulus of rupture is 119–135 N/mm², modulus of elasticity 10,380–12,250 N/mm², compression parallel to grain 39–48 N/mm², cleavage 19–20.5 N/mm, Janka side hardness 5200 N, Janka end hardness 6490 N and Chalais-Meudon side hardness 1.7–3.8.
The wood saws fairly easy, planes well and takes a good polish. It holds nails and screws well, glues well, and makes good joints. The wood is durable even when exposed to the soil or water, being quite resistant to fungi and all kinds of insect attacks including termites, but the sapwood is susceptible to Lyctus attack. It is an excellent firewood and produces a good-quality charcoal.
The wood contains about 43% cellulose, 28% lignin, 14% pentosan, 1.1% ash and little silica. The solubility is 6.6% in alcohol-benzene, 2.1% in hot water and 14.1% in a 1% NaOH solution.
Tannins isolated from the bark showed antibacterial activity, and aqueous bark extracts showed anti-inflammatory effect. Methanolic and aqueous extracts of roots and stem bark of Bridelia micrantha were shown to have strong activity against HIV-1 reverse transcriptase and integrase. Methanolic bark extracts showed in-vitro inhibition of a wide range of gram-positive and gram-negative bacteria. Extracts of the roots had the same effect but only at higher concentrations. Compounds isolated and possibly responsible for the antibacterial activity are friedelin, taraxerone, epifriedelinol, taraxerol and the tannins gallic acid, ellagic acid and caffeic acid.
Adulterations and substitutes
The wood resembles that of Lovoa trichilioides Harms. The timber of Bridelia micrantha shares its trade name with that of Bridelia grandis Pierre ex Hutch. and the timber of both species is mixed in trade.
Description
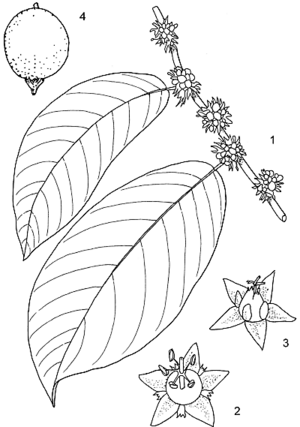
- Evergreen or deciduous, monoecious, small to medium-sized tree up to 20(–27) m tall; bole short, often twisted, up to 50(–100) cm in diameter, sometimes with scattered blunt spines; bark surface with fine fissures, dark grey; crown spreading, fairly open; branches often with blunt spines; twigs sparingly soft-hairy or glabrous.
- Leaves alternate, distichous, simple and entire; stipules linear-lanceolate, 4–7 mm long, caducous; petiole 5–13 mm long; blade elliptical to oblong-elliptical, 3–28 cm × 2–12 cm, base rounded to cuneate, apex short-acuminate, glabrous to slightly hairy above, sparingly soft-hairy below, pinnately veined with 5–20 pairs of lateral veins.
- Inflorescence a small axillary fascicle.
- Flowers unisexual, regular, 5(–6)-merous; sepals triangular, c. 2 mm long; petals small, 0.5–1 mm long; disk shallowly 5-lobed; male flowers with pedicel c. 1 mm long, stamens with filaments fused in a column below, free and spreading above, ovary rudimentary; female flowers nearly sessile with superior, 2–3-celled ovary, styles 2, fused at base, 2-branched.
- Fruit a nearly globose, fleshy drupe c. 7 mm in diameter, black when ripe, 1-seeded.
- Seeds c. 5 mm long, brownish.
- Seedling with epigeal germination; hypocotyl 3–4 cm long, epicotyl 0.5–1 cm long, slightly hairy; cotyledons leafy, transversely oblong, c. 1 cm × 2 cm; first leaves alternate.
Other botanical information
Bridelia occurs in the Old World tropics, and comprises about 75 species. About 15 species occur in mainland tropical Africa and 2 species are endemic to the Indian Ocean islands.
Bridelia ndellensis
Bridelia ndellensis Beille is a small tree up to 15(–20) m tall with bole up to 30 cm in diameter, distributed from southern Nigeria eastward to Sudan and Uganda. Its wood is whitish and hard, and used in DR Congo for house building. In Sudan the fruits are eaten. In DR Congo a bark maceration is taken as a remedy for cough and diarrhoea.
Anatomy
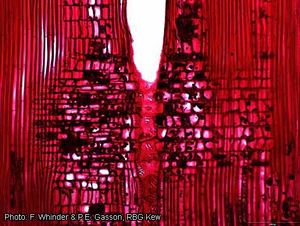
Wood-anatomical description (IAWA hardwood codes):
- Growth rings: 1: growth ring boundaries distinct; 2: growth ring boundaries indistinct or absent.
- Vessels: 5: wood diffuse-porous; 13: simple perforation plates; (19: reticulate, foraminate, and/or other types of multiple perforation plates); 22: intervessel pits alternate; 23: shape of alternate pits polygonal; 25: intervessel pits small (4–7 μm); 26: intervessel pits medium (7–10 μm); (30: vessel-ray pits with distinct borders; similar to intervessel pits in size and shape throughout the ray cell); 31: vessel-ray pits with much reduced borders to apparently simple: pits rounded or angular; 32: vessel-ray pits with much reduced borders to apparently simple: pits horizontal (scalariform, gash-like) to vertical (palisade); (33: vessel-ray pits of two distinct sizes or types in the same ray cell); (34: vessel-ray pits unilaterally compound and coarse (over 10 μm)); 42: mean tangential diameter of vessel lumina 100–200 μm; 47: 5–20 vessels per square millimetre; 56: tyloses common.
- Tracheids and fibres: 61: fibres with simple to minutely bordered pits; 65: septate fibres present; 66: non-septate fibres present; 69: fibres thin- to thick-walled.
- Axial parenchyma: 76: axial parenchyma diffuse; 78: axial parenchyma scanty paratracheal; 92: four (3–4) cells per parenchyma strand; 93: eight (5–8) cells per parenchyma strand.
- Rays: 97: ray width 1–3 cells; (98: larger rays commonly 4- to 10-seriate); 107: body ray cells procumbent with mostly 2–4 rows of upright and/or square marginal cells; 108: body ray cells procumbent with over 4 rows of upright and/or square marginal cells; 109: rays with procumbent, square and upright cells mixed throughout the ray; (110: sheath cells present); 115: 4–12 rays per mm.
- Mineral inclusions: 136: prismatic crystals present; 137: prismatic crystals in upright and/or square ray cells; 138: prismatic crystals in procumbent ray cells; 140: prismatic crystals in chambered upright and/or square ray cells; 141: prismatic crystals in non-chambered axial parenchyma cells; 142: prismatic crystals in chambered axial parenchyma cells.
Growth and development
Under favourable conditions when planted in deep and moist soils, growth of seedlings is fast, up to 2 m per year. Bridelia micrantha already produces considerable shade and may start flowering 3 years after sowing. Flowering takes place during the dry season. Reportedly, trees do not fruit every year. Fruits are dispersed by numerous animals, including civet, rodents and probably birds. Roots may produce suckers when they are injured.
Ecology
Bridelia micrantha is found in a variety of habitats, from savanna and woodland to seasonally flooded grassland, riverine forest, swamp forest and the margins of mangrove swamps, from sea-level in West Africa to 1750(–2500) m altitude in East Africa. It is a pioneer that tolerates a wide diversity of soils. It withstands moderate frost.
Propagation and planting
Sowing fresh seed is the easiest way to propagate Bridelia micrantha. The oily seeds lose their viability quickly and storing seed for longer periods is difficult. Wildlings are also collected for planting. In Uganda cuttings have been successfully used to establish plantations. For good early growth, weeding is necessary after planting.
Management
Bridelia micrantha can be pollarded, pruned and coppiced, and a 30 year coppicing rotation has been proposed. In small farms in Tanzania, it is commonly intercropped and pollarded in short rotations to reduce shade from the crowns. In Nigeria and Uganda pure stands have been established for the production of silkworms.
Genetic resources
In Ethiopia Bridelia micrantha has become scarce as a result of overexploitation for its termite-resistant wood. In Kenya the intensive use has had the same effect and probably populations are under pressure in many other regions. To have continued access to sizeable timber, protective measures and domestication are needed.
Prospects
There will be continued demand for the wood of Bridelia micrantha for applications where durability is demanded. The isolation and the structural elucidation of the active constituents of Bridelia micrantha will provide useful leads in the development of antibiotics with β-lactamase inhibitory activity. Resistance of bacteria to this type of antibiotics is rare. For the production of silk the prospects are good. Research into domestication and management practices is important for all applications.
Major references
- Arbonnier, M., 2004. Trees, shrubs and lianas of West African dry zones. CIRAD, Margraf Publishers Gmbh, MNHN, Paris, France. 573 pp.
- Bekele-Tesemma, A., 2007. Useful trees and shrubs for Ethiopia: identification, propagation and management for 17 agroclimatic zones. Technical Manual No 6. RELMA in ICRAF Project, Nairobi, Kenya. 552 pp.
- Bolza, E. & Keating, W.G., 1972. African timbers: the properties, uses and characteristics of 700 species. Division of Building Research, CSIRO, Melbourne, Australia. 710 pp.
- Burkill, H.M., 1994. The useful plants of West Tropical Africa. 2nd Edition. Volume 2, Families E–I. Royal Botanic Gardens, Kew, Richmond, United Kingdom. 636 pp.
- de Koning, J., 1983. La forêt de Banco. Part 2: La Flore. Mededelingen Landbouwhogeschool Wageningen 83–1. Wageningen, Netherlands. 921 pp.
- Léonard, J., 1962. Euphorbiaceae. In: Robyns, W., Staner, P., Demaret, F., Germain, R., Gilbert, G., Hauman, L., Homès, M., Jurion, F., Lebrun, J., Vanden Abeele, M. & Boutique, R. (Editors). Flore du Congo belge et du Ruanda-Urundi. Spermatophytes. Volume 8, 1. Institut National pour l’Étude Agronomique du Congo belge, Brussels, Belgium. 214 pp.
- Neuwinger, H.D., 2000. African traditional medicine: a dictionary of plant use and applications. Medpharm Scientific, Stuttgart, Germany. 589 pp.
- Radcliffe-Smith, A., 1996. Euphorbiaceae, subfamilies Phyllantoideae, Oldfieldioideae, Acalyphoideae, Crotonoideae and Euphorbioideae, tribe Hippomaneae. In: Pope, G.V. (Editor). Flora Zambesiaca. Volume 9, part 4. Royal Botanic Gardens, Kew, Richmond, United Kingdom. pp. 1–337.
- Raponda-Walker, A. & Sillans, R., 1961. Les plantes utiles du Gabon. Paul Lechevalier, Paris, France. 614 pp.
- Takahashi, A., 1978. Compilation of data on the mechanical properties of foreign woods (part 3) Africa. Shimane University, Matsue, Japan. 248 pp.
Other references
- Bessong, P.O., Obi, C.L., Andréola, M.L., Rojas, L.B., Pouységu, L., Igumbor, E., Marion Meyer, J.J., Quideau, S. & Litvak, S., 2005. Evaluation of selected South African medicinal plants for inhibitory properties against human immunodeficiency virus type 1 reverse transcriptase and integrase. Journal of Ethnopharmacology 99: 83–91.
- Chiotha, S.S., Seyani, J.H. & Fabiano, E.C., 1991. Molluscicidal and piscicidal properties of indigenous plants. In: Pierce, B.A.C., Lightfoot, C., Ruddle, K. & Pullin, R.S.V. (Editors). Aquaculture research and development in rural Africa. ICLARM Conference Proceedings 27. 71 pp.
- Dalziel, J.M., 1937. The useful plants of West Tropical Africa. Crown Agents for Overseas Governments and Administrations, London, United Kingdom. 612 pp.
- de la Mensbruge, G., 1966. La germination et les plantules des essences arborées de la forêt dense humide de la Côte d’Ivoire. Centre Technique Forestier Tropical, Nogent-sur-Marne, France. 389 pp.
- Gangoué-Piéboji, J., Pegnyemb, D.E., Niyitegeka, D., Nsangou, A., Eze, N., Minyem, C., Mbing, J.N., Ngassam, P., Tih, R.G., Sodengam, B.L. & Bodo, B., 2006. The in-vitro antimicrobial activities of some medicinal plants from Cameroon. Annals of Tropical Medicine and Parasitology 100(3): 237–243.
- Gangoué-Piéboji, J., Baurin, S., Frère, J.M., Ngassam, P., Ngameni, B., Azebaze, A., Pegnyemb, D.E., Watchueng, J., Goffin, C. & Galleni, M., 2007. Screening of some medicinal plants from Cameroon for ß-Lactamase inhibitory activity. Phytotherapy Research 21(3): 284–287.
- Gillah, P.R., Ishengoma, R.C., Amartey, S.A., Gabriel, J., Kitojo, D.H. & Negi, A., 2004. Natural durability of some lesser-known timber species against rotting fungi. Journal of the Timber Development Association of India 50(3/4): 32–41.
- Guéneau, P., Bedel, J. & Thiel, J., 1970–1975. Bois et essences malgaches. Centre Technique Forestier Tropical, Nogent-sur-Marne, France. 150 pp.
- Johnson, D. & Johnson, S., 2002. Down to Earth: Gardening with indigenous trees. Struik Publishers, Cape Town, South Africa. 112 pp.
- Mbahin, N., Raina, S.K., Kioko, E.N. & Mueke, J.M., 2008. Spatial distribution of cocoon nests and egg clusters of the silkmoth Anaphe panda (Lepidoptera: Thaumetopoeidae) and its host plant Bridelia micrantha (Euphorbiaceae) in the Kakamega Forest of western Kenya. International Journal of Tropical Insect Science 27(3-4): 138–144.
- Mbahin, N., Raina, S.K., Kioko, E.N. & Mueke, J.M., 2010. Use of sleeve nets to improve survival of the Boisduval silkworm, Anaphe panda, in the Kakamega Forest of western Kenya. Journal of Insect Science 10(6): 1–10.
- Moshi, M.J. & Mbwambo, Z.H., 2002. Experience of Tanzanian traditional healers in the management of non-insulin dependent diabetes mellitus. Pharmaceutical Biology 40(7): 552–560.
- Mushambanyi, T.M.B., 2000. Etude préliminaire orientée vers la production des chenilles consommables par l'élevage des papillons (Anaphe infracta: Thaumetopoeidae) à Lwiro, Sud-Kivu. République Démocratique du Congo. Tropicultura 18(4): 208–211.
- Ngueyem, T.A., Brusotti, G., Caccialanza, G. & Vita Finzi, P., 2009. The genus Bridelia: a phytochemical and ethnopharmacological review. Journal of Ethnopharmacology 124(3): 339–349.
- Noad, T. & Birnie, A., 1989. Trees of Kenya. A fully illustrated field guide. Nairobi, Kenya. 281 pp.
- Sallenave, P., 1955. Propriétés physiques et mécaniques des bois tropicaux de l’Union française. Centre Technique Forestier Tropical, Nogent-sur-Marne, France. 129 pp.
- Samie, A., Obi, C.L., Bessong, P.O. & Namrita, L., 2005. Activity profiles of fourteen selected medicinal plants from rural Venda communities in South Africa against fifteen clinical bacterial species. African Journal of Biotechnology 4(12): 1443–1451.
- SEPASAL, 2011. Bridelia micrantha. [Internet] Survey of Economic Plants for Arid and Semi-Arid Lands (SEPASAL) database. Royal Botanic Gardens, Kew, Richmond, United Kingdom. http://www.kew.org/ ceb/sepasal/. November 2011.
- Teel, W., 1984. A pocket directory of trees and seeds in Kenya. Kenya Energy Non-Governmental Organisations, Nairobi, Kenya. 151 pp.
- Watt, J.M. & Breyer-Brandwijk, M.G., 1962. The medicinal and poisonous plants of southern and eastern Africa. 2nd Edition. E. and S. Livingstone, London, United Kingdom. 1457 pp.
Sources of illustration
- Akoègninou, A., van der Burg, W.J. & van der Maesen, L.J.G. (Editors), 2006. Flore analytique du Bénin. Backhuys Publishers, Leiden, Netherlands. 1034 pp.
Author(s)
- C.H. Bosch, PROTA Network Office Europe, Wageningen University, P.O. Box 341, 6700 AH Wageningen, Netherlands
Correct citation of this article
Bosch, C.H., 2012. Bridelia micrantha (Hochst.) Baill. [Internet] Record from PROTA4U. Lemmens, R.H.M.J., Louppe, D. & Oteng-Amoako, A.A. (Editors). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. <http://www.prota4u.org/search.asp>.
Accessed 18 December 2024.
- See the Prota4U database.