Sinarundinaria alpina (PROTA)
Introduction |
| General importance | |
| Geographic coverage Africa | |
| Geographic coverage World | |
| Vegetable | |
| Timber | |
| Fuel | |
| Forage / feed | |
| Auxiliary plant | |
| Food security | |
Sinarundinaria alpina (K.Schum.) C.S.Chao & Renvoize
- Protologue: Kew Bull. 44(2): 361 (1989).
- Family: Poaceae (Gramineae)
Synonyms
- Arundinaria alpina K.Schum. (1895),
- Yushania alpina (K.Schum.) W.C.Lin (1974).
Vernacular names
- African alpine bamboo, mountain bamboo (En).
- Bambou creux (Fr).
- Mianzi, mwanzi (Sw).
Origin and geographic distribution
African alpine bamboo occurs in scattered populations on mountains from southern Sudan and Ethiopia southwards to Malawi. An interval of 2000 km separates its occurrence in western Cameroon from those in eastern Africa. It is frequently planted, e.g. in Ethiopia and Zimbabwe.
Uses
The whole stems of African alpine bamboo are used for hut construction, particularly as rafters, and for fencing. In the Poroto mountains in Tanzania entire villages are constructed using this bamboo, and water pipes made from African alpine bamboo supplied water to around 100,000 people in the 1980s. A few specialized enterprises produce bamboo furniture. Split canes are woven into storage pots and baskets in Tanzania and Kenya; in Uganda they are made into beehives. Dry stems are used as fuel. Young shoots are marketed as a vegetable (Mount Elgon, Kenya/Uganda border), and leaves and fine branchlets serve as cattle fodder. Sinarundinaria alpina is also valued in soil conservation as a more effective watershed cover than trees.
Production and international trade
African alpine bamboo forest is most extensive in the high mountains of the Nile/Congo divide and further east (500,000 ha, with over 100,000 ha in each of DR Congo, Ethiopia and Kenya). In Cameroon the area is less than 20,000 ha. Its gregarious character typically results in a standing crop of more than 5000 full-sized stems per ha in mixtures with Afromontane trees, and up to 40,000 stems per ha in pure stands. There is no international trade based on the species, but some commerce exists in the countries where it grows. Levels of utilization reported in national statistics, based on payments for harvesting licences, underestimate exploitation for immediate local community needs.
Properties
The mean density of the stem wall of African alpine bamboo is about 0.7 g/cm³ at 8% moisture content. Dried stems used in construction and fencing are susceptible to infestation by the powder-post beetle Dinoderus minutus. Nevertheless, the stems are considered durable and houses and fences made from them in DR Congo are said to last for more than 20 years.
Stems from DR Congo contained: holocellulose 60–65%, α-cellulose 49%, pentosans 17%, lignin 24%, ash 3%. Solubilities were 3.6% (hot water), 1.8% (alcohol-benzene) and 22.2% (1% NaOH). The mean fibre length was 2.0 mm, with a diameter of 17.9 μm, a lumen width of 3.6 μm and a cell wall thickness of 7.2 μm. Stems give reasonable yields of readily bleached pulp suitable for producing writing and printing paper.
The foliage is fibrous (crude fibre 26.6%) and rich in ash (15.3%). Analysis of material from Ethiopia (Masha) indicates that stems are low in N, particularly those more than 3 years old (0.3%), but in leaves/branchlets the level is higher (1.8%). Rhizomes are relatively rich in potassium (1.4%) and leaves/branchlets much richer in calcium (0.3%) than other parts of the plant (0.02–0.06%). Phosporus levels are fairly uniform throughout the plant (0.05–0.16%).
Description
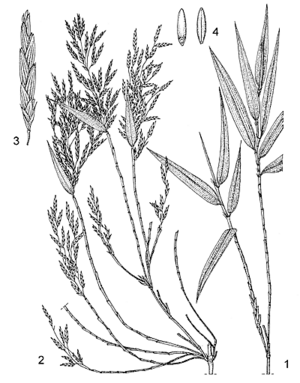
- Evergreen bamboo with short rhizome up to 10 cm thick, and usually not closely clustered stems; stem (culm) erect, up to 20 m tall, up to 12.5 cm in diameter, yellowish at maturity, hollow, thick-walled, many-noded, profusely branched.
- Leaves alternate, simple; stem leaves with sheath ovate to oblong-lanceolate, glabrous or with reddish brown bristly hairs, tipped with a linear blade c. 6 cm long; branch leaves with sheath having small lateral auricles, ligule c. 2 mm long, blade linear-lanceolate to narrowly lanceolate, 5–20 cm × 0.5–1.5 cm, base constricted into a short stalk, apex acute or with apical bristle up to 2 cm long, glaucous to bright green, glabrous, with conspicuous transverse veins.
- Inflorescence a terminal panicle 5–15 cm long.
- Spikelets linear to linear-lanceolate, 15–50 mm × 3–4 mm, comprising 4–11 florets, the apical ones sterile; glumes lanceolate or narrowly ovate, 4–8 mm long, apex acuminate, papery, 5–9-veined, persistent; lemma lanceolate-oblong to ovate, 7–10 mm long, apex acute, 7–9-veined, palea linear-oblong, about as long as lemma, apex truncate, 7–9-veined; florets with 3 stamens, 3 lodicules, and glabrous ovary with 2 stigmas.
- Fruit a spindle-shaped caryopsis (grain) 1.5–6 mm long, blackish brown, with a longitudinal furrow.
Other botanical information
Sinarundinaria comprises about 50 species, most of them from tropical Asia, 2 in Central America, 1 in mainland Africa and 2 in Madagascar. Sinarundinaria alpina has been included in several genera, but in recent molecular phylogenetic analysis its exact position within the so-called Thamnocalamus group and allies remained unclear. Additional research is needed.
In the past, small individuals of African alpine bamboo have occasionally been misidentified as Thamnocalamus tessellatus (Nees) Soderstr. & R.P.Ellis, the South African mountain bamboo. Thamnocalamus tessellatus differs in having thin-walled stems arising from elongated rhizomes, stem leaves with lanceolate blades and without auricles, and branch leaves with hard acuminate tips and hairy ligule, whereas its spikelets are sessile and ovate, and contain only a single fertile floret.
Growth and development
Although nursery seedlings have been raised in Kenya, there is no published description of the germination and initial growth of African alpine bamboo. Small plants, consisting entirely of new shoots, form loose, but discrete, clumps. With time, stems at the centre die and disappear, while new stems are added at the periphery. Repeated exposure to fire causes mortality of young peripheral rhizomes and concentration of new shoots close to older ones, maintaining relatively compact clumps. Rhizome networks producing new stems annually may survive for at least 40 years, but individual stems survive only 8–14 years. New stem production is a seasonal process, allowing cohorts of stems in different age classes to be recognized. A year of vigorous stem production may be followed by a 2–3-year period of low stem production. Unusual droughts also reduce production in the subsequent growing season. The annual growth cycle involves the development of new stems at rhizome apices where growth is activated when the rainy season starts. New stems reach full height in 2–4 months and branch in the following year. By the fourth year of growth the stems are glabrous and sufficiently rigid for use as poles. Estimates of the interval between flowering events vary from 15 years (Mount Elgon, Kenya) to 40 years (Aberdares Range, Kenya). Flowering may be synchronous in patches several hectares in extent within a population. It is generally assumed that plants die after flowering, although development of new shoots from parts of the rhizome network surviving after flowering has been noted in Kenya.
Ecology
Sinarundinaria alpina is restricted to high elevations (2000–4000 m altitude) and is the characteristic and definitive dominant of Afromontane bamboo vegetation. It also occurs in abandoned fields and it can form extensive pure stands. Afromontane bamboo vegetation occurs in cool growing conditions, with average annual temperatures of 14–17°C. Average monthly maximum temperatures are 13–32°C, and average monthly minimum temperatures range from –4°C to 11°C, implying that some populations tolerate frost. Rainfall is seasonal, with 3–6 dry months (mean rainfall less than 50 mm) in eastern Africa, but only 2 dry months in Cameroon. Annual totals vary from 800 mm in Tanzania to 2000 mm in Ethiopia and 3000 mm in Cameroon. Climate requirements over-ride soil type requirements, with occurrences on impoverished ferralsols, moderately fertile cambisols, and richer andosols and nitisols. Well-drained humus-rich soil on gentle slopes and in ravines, with space for vigorous rhizome development, allows luxuriant growth. On shallow soils and rocky ground individuals are stunted. Afromontane bamboo forest has been described as a climax formation, but burnt forest tree stumps within Sinarundinaria alpina stands have been interpreted as evidence of a fire-induced community. Another view treats the species as a light-demanding pioneer forming populations maintained by large herbivore activity.
Propagation and planting
Attempts to germinate seed of African alpine bamboo from the few seed crops occurring are not always successful, but in Kenya seeds sown in nursery beds and watered daily have germinated. Seedlings 2–3 cm tall were transferred to soil boxes and planted out 8–12 months later, at 2 m spacing. A stand with stems up to 12 m tall and 5 cm in diameter resulted after 6 years.
In parts of Ethiopia and Uganda offsets are often planted. In experiments in Kenya, there has been successful use of offsets (single stems shortened to 60 cm, with attached rhizome), clump division (groups of 5 stems shortened to 60 cm, with the parent rhizome) and 20 cm lengths of rhizome. Offsets with stems produced in the previous growing season are preferred. Stem cuttings have not produced shoots, not even after treatment with rooting compounds.
Management
Management of African alpine bamboo is mainly limited to harvesting of natural stands, undertaken by area rather than by clump, because stems are usually well separated. For Kenya, burning after exploitation has been recommended. For nitisols in the Masha Forest in Ethiopia, applications of P and K at the end of the rainy season have been recommended to sustain productivity.
Diseases and pests
Association of African alpine bamboo with the basidiomycete Armillaria mellea has been reported in Kenya and there is suspicion that the fungus spreads from a reservoir in the bamboo to planted pines and hardwood trees.
Harvesting
Natural stands of African alpine bamboo can be clear-felled but recovery is slow, development of full-sized stems taking 9–10 years. Stems must be full-sized and at least 3 years old before they can be exploited for structural use, and numbers must accumulate to levels making harvesting worthwhile, so felling cycles of 14–21 years have been recommended. The cycle can be reduced to 5–6 years on good sites if modest yields are acceptable and 50% of the mature stems are retained. Harvesting of stems under 2 years old suitable for weaving, and harvesting of edible shoots as vegetable are rainy season activities.
Yield
The dry weight of a standing crop of stems of well-stocked stands in Masha, Ethiopia has been estimated at 51 t/ha and in Kenya at 97 t/ha. With a 5-year cutting cycle exploiting only mature stems, representing 20% of the number of stems present, a potential yield of 10 t/ha per year has been estimated for Ethiopia. Better management might raise this to 15 t/ha per year.
Handling after harvest
Stems for construction are stripped of branches and trimmed to lengths of 7.5–9 m. Drying and protection against the powder-post beetle Dinoderus minutus attack are advisable. Protection of complete stems with preservative solutions is difficult but it is assumed that soaking in water for 2–3 months, widely used with other bamboos, may provide some protection.
In Uganda internodes of stems harvested for weaving material are cut into slivers which may be bundled and stored for several months before use. Edible shoots are sun-dried or smoked and can be stored for up to 2 years.
Genetic resources
Today’s distribution of Sinarundinaria alpina, including intervals well in excess of 100 km, makes geographic differences probable. There are no germplasm collections, however, although ex-situ cultivation is reported for the Muguga Arboretum, Kenya. Reports at national level indicate population losses and conservation threats. The population of Chimaliro, Malawi, is thought to be recently extinct. Other populations have shrunk, with causes suggested being successional vegetation change following fire, exclusion and elimination of elephants and buffaloes (Echuya, Uganda), conversion to agricultural land (Ethiopia) and over-exploitation of the stems (Tanzania, Uganda).
Prospects
African alpine bamboo is of high local importance throughout its range, and in Ethiopia, Kenya, Tanzania and Uganda there is a consistent small supply of bamboo material and products to markets remote from the source areas. The International Network for Bamboo and Rattan (INBAR) has evaluated bamboo ‘production to consumption’ systems in these 4 countries. Exploitation is not subject to effective regulation and monitoring, and declared policies on national bamboo resources are lacking. There is little professional expertise to promote active management and refine harvesting to obtain shoots as well as young and mature poles, or encourage the development of processing and marketing frameworks. Nevertheless, enough silvicultural knowledge exists for preliminary management schedules to be applied, taking account of the age structure of the stands in the context of marketable products and harvesting intensity and frequency. The infrequent flowering and uncertain seed viability invite development of an economically attractive method of vegetative propagation, for stand rehabilitation and wider planting on agricultural land. The distinctive dominance, or even purity, of the species where it grows, and the short-rotation potential justify further research into prospects for bamboo material entering the pulp/paper industry if demand exists and if sufficient material of quality comparable or superior to current alternatives can be sustainably supplied. Clarification of geographic variation through co-ordinated systematic study could reveal provenances of differing quality and relevance for improvement initiatives.
Major references
- Alvino, G.E., 1950. La foresta di bambù alpino nell’Africa orientale. Proceedings of the Third World Forestry Congress (10–20 July 1949, Helsinki), 3 (Special Papers): 11–17.
- Clayton, W.D., 1970. Gramineae (part 1). In: Milne-Redhead, E. & Polhill, R.M. (Editors). Flora of Tropical East Africa. Crown Agents for Oversea Governments and Administrations, London, United Kingdom. 176 pp.
- Clayton, W.D., Harman, K.T. & Williamson, H., 2002–. GrassBase - the online world grass flora. [Internet] Rotal Botanic Gardens, Kew, United Kingdom.http://www.kew.org/ data/grasses-db/. October 2007.
- Embaye, K., Weih, M., Ledin, S. & Christersson, L., 2005. Biomass and nutrient distribution in a highland bamboo forest in southwest Ethiopia: implications for management. Forest Ecology and Management 204(2–3): 159–169.
- Hemp, A., 2006. Vegetation of Kilimanjaro: hidden endemics and missing bamboo. African Journal of Ecology 44: 305–328.
- Hubbard, C.E., 1962. Arundinaria alpina K.Schum. Hooker’s Icones Plantarum (ser. 5) 6: 1–6.
- Istas, J.R. & Raekelboom, E.L., 1962. Etude biométrique, chimique et papetière des bambous du Congo. Série Technique No 67. Institut National pour l’Etude Agronomique du Congo (INEAC), Brussels, Belgium. 53 pp.
- Kigomo, B.N. & Kamiri, J.F., 1987. Studies in propagation and establishment of Oxytenanthera abyssinica, Bambusa vulgaris and Arundinaria alpina in medium altitude site in Kenya. Kenya Journal of Sciences (B) 8: 5–13.
- Launert, E., 1971. Gramineae (Bambuseae - Pappophoreae). In: Fernandes, A., Launert, E. & Wild, H. (Editors). Flora Zambesiaca. Volume 10, part 1. Flora Zambesiaca Managing Committee, London, United Kingdom. 152 pp.
- Wimbush, S.H., 1945. The African alpine bamboo. Empire Forestry Journal 24: 33–39.
Other references
- Ayre-Smith, R.A., 1963. The use of bamboo as a cattle feed. East African Agricultural and Forestry Journal 29: 50–51.
- Banana, A.Y. & Tweheyo, M., 2001. The ecological changes of Echuya afromontane bamboo forest, Uganda. African Journal of Ecology 39: 366–373.
- Banana, A.Y. & Tweheyo, M., 2004. Ecological changes following rules in use and anthropology: the case of Echuya bamboo forest, south-western Uganda. Uganda Journal 50: 39–49.
- Chao, C.-S. & Renvoize, S.A., 1989. A revision of the species described under Arundinaria (Gramineae) in Southeast Asia and Africa. Kew Bulletin 44(2): 349–367.
- CTFT (Centre Technique Forestier Tropical), 1962. Bambous en Afrique (Arundinaria alpina, Bambusa vulgaris, Oxytenanthera abyssinica). Bois et Forêts des Tropiques 85: 24–32.
- Cunningham, R.L., Clark, T.F., Kwolek, W.F., Wolff, I.A. & Jones, Q., 1970. A search for new fiber crops. XIII. Laboratory-scale pulping studies continued. Tappi Journal 53: 1697–1700.
- Esegu, J.F., Ssenteza, J. & Sekatuba, J., 2000. Rattan and bamboo in Uganda: a study of the production to consumption systems. INBAR Working Paper 29: 1–15.
- Gibson, I.A.S., 1960. Armillaria mellea in Kenya forests. East African Agricultural and Forestry Journal 26: 142–143.
- Guo, Z.-H. & Li, D.-Z., 2004. Phylogenetics of the Thamnocalamus group and its allies (Gramineae: Bambusoideae): inference from the sequences of GBSSI gene and ITS spacer. Molecular Phylogenetics and Evolution 30: 1–12.
- Kelbessa, E., Bekele, T., Gebrihiwot, A. & Hadera, G., 2000. A socio-economic case study of the bamboo sector in Ethiopia: an analysis of the production-to-consumption system. INBAR Working Paper 25: 1–20.
- Kigomo, B.N., 1990. Bamboo resource in the East African region. In: Rao, I.V.R., Gnanaharan, R. & Sastry, C.B. (Editors). Bamboos: current research. Proceedings of the international bamboo workshop held in Cochin, India from 14–18 November 1988. Kerala Forest Research Institute, Peechi, Kerala, India & International Development Research Centre, Ottawa, Canada. pp. 22–28.
- Li, D.-Z., 1997. The Flora of China Bambusoideae Project - problems and current understanding of bamboo taxonomy in China. In: Chapman, G.R. (Editor). The bamboos. Academic Press, London, United Kingdom. pp. 61–81.
- Lovett, J.C., Ruffo, C.K., Gereau, R.E. & Taplin, J.R.D., 2006. Field guide to the moist forest trees of Tanzania. [Internet] Centre for Ecology Law and Policy, Environment Department, University of York, York, United Kingdom. http://www.york.ac.uk/ res/celp/webpages/projects/ecology/ tree%20guide/guide.htm. October 2007.
- Mgeni, A.S.M. & Swai, C.M.S., 1982. Bamboo water pipes. Commonwealth Forestry Review 61(4): 285–286.
- Ohrnberger, D., 1999. The bamboos of the World. Elsevier, Amsterdam. 585 pp.
- Ongugo, P.O., Sigu, G.O., Kariuki, J.G., Luvanda, A.M. & Kigomo, B.N., 2000. Production-to consumption systems: a case study of the bamboo sector in Kenya. INBAR Working Paper 27: 1–60.
- Phillips, S., 1995. Poaceae (Gramineae). In: Hedberg, I. & Edwards, S. (Editors). Flora of Ethiopia and Eritrea. Volume 7. Poaceae (Gramineae). The National Herbarium, Addis Ababa University, Addis Ababa, Ethiopia and Department of Systematic Botany, Uppsala University, Uppsala, Sweden. 420 pp.
- Snowden, J.D., 1953. The grass communities and mountain vegetation of Uganda. Crown Agents, London, United Kingdom. 94 pp.
- Soderstrom, T.R. & Ellis, R.P., 1982. Taxonomic status of the endemic South African bamboo, Thamnocalamus tessellatus. Bothalia 14: 53–67.
- van der Zon, A.P.M., 1992. Graminées du Cameroun. Volume 2, Flore. Wageningen Agricultural University Papers 92–1. Wageningen Agricultural University, Wageningen, Netherlands. 557 pp.
Sources of illustration
- Clayton, W.D., 1970. Gramineae (part 1). In: Milne-Redhead, E. & Polhill, R.M. (Editors). Flora of Tropical East Africa. Crown Agents for Oversea Governments and Administrations, London, United Kingdom. 176 pp.
- Engler, A., 1908. Die Pflanzenwelt Afrikas insbesondere seiner tropischen Gebiete. Grundzüge der Pflanzenverbreitung in Afrika und die Charakterpflanzen Afrikas. Band 2. Wilhelm Engelmann, Leipzig, Germany. 460 pp.
Author(s)
- J.B. Hall, School of Agricultural and Forest Sciences, University of Wales, Bangor, Gwynedd LL57 2UW, United Kingdom
- T. Inada, 3-21-12, Toyotamanaka, Nerimaku, Tokyo, 176-0013, Japan
Correct citation of this article
Hall, J.B. & Inada, T., 2008. Sinarundinaria alpina (K.Schum.) C.S.Chao & Renvoize. In: Louppe, D., Oteng-Amoako, A.A. & Brink, M. (Editors). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. Accessed 17 December 2024.
- See the Prota4U database.