Acacia mearnsii (PROTA)
Introduction |
| General importance | |
| Geographic coverage Africa | |
| Geographic coverage World | |
| Dye / tannin | |
| Medicinal | |
| Timber | |
| Fuel | |
| Ornamental | |
| Forage / feed | |
| Auxiliary plant | |
| Fibre | |
| Climate change | |
Acacia mearnsii De Wild.
- Protologue: Pl. bequaert. 3(1): 61 (1925).
- Family: Mimosaceae (Leguminosae - Mimosoideae)
- Chromosome number: 2n = 26
Synonyms
- Racosperma mearnsii (De Wild.) Pedley (1986),
- Acacia decurrens auct. non Willd.,
- Acacia mollissima auct. non Willd.
Vernacular names
- Black wattle, tan wattle (En).
- Acacia noir (Fr).
- Acácia negra (Po).
- Muwati (Sw).
Origin and geographic distribution
Black wattle is native to south-eastern Australia from 35–44°S latitude (New South Wales, Queensland, Victoria and Tasmania). It has been introduced throughout the tropics and subtropics. Large commercial plantations are found in southern and eastern Africa (Kenya, South Africa and Zimbabwe), Brazil and India. Elsewhere plantings are smaller or introductions have not been successful.
Uses
Black wattle is primarily cultivated for tannin and wood production. It is the world’s principal source of tanbark; the bark contains up to 40% of excellent tannin especially suitable for use in the manufacture of heavy leather goods. In addition, the powdered bark extract is used to prepare tannin formaldehyde adhesives for exterior grade plywood, particle board and laminated timber. Possibilities of using the bark in the production of biodegradable polyurethane foam are being tested. The wood of the tree is widely used as fuelwood for domestic use and village industries, or for charcoal production (e.g. in Kenya, South Africa and Brazil). The wood may also be used for local construction material, mine props, wooden tools, joinery, flooring and hardboard. Thin, pliable stems are used in the framework of traditional wattle and daub huts by many African people. The wood is used in combination with other woods to produce paper pulp and dissolving (or viscose) pulp, the raw material used to produce synthetic fibres such as rayon. In recent years the use of black wattle wood in the pulp and paper industry has increased substantially worldwide. Japan in particular is a large importer of black wattle chips from South Africa. Black wattle is also planted for erosion control and soil improvement, as shelter belts or fire belts, as a shade tree in plantations, and as an ornamental. The leaves are sometimes used for fodder, but are relatively unpalatable and can best be mixed with other feeds. A decoction of the very astringent bark is used as a styptic and to treat diarrhoea.
Production and international trade
The maximum area of black wattle plantations was reached around the 1960s. Since then, a fall in demand for vegetable tannin has led to a considerable reduction in area, e.g. from 325,000 ha to 130,000 ha in South Africa and from 27,000 ha to 14,000 ha in Zimbabwe. Around 1980 the estimated plantation area was about 350,000 ha, of which 160,000 ha were in South Africa, 125,000 ha in Brazil, 30,000 ha in East Africa (Zimbabwe, Kenya, Tanzania, Rwanda, Burundi), 20,000 ha in India, and 15,000 ha in Indonesia. Black wattle is currently the world’s major source of vegetable tannin. In several countries, including Kenya, Zimbabwe and South Africa, tannin industries based on black wattle have been developed. South Africa also produces a variety of adhesives from the bark extracts. The main exporting countries are South Africa (30,000 t/year of tan extract and 15,000 t/year of adhesive products) and Kenya (25,000 t/year of tan extract, but the Kenyan factory has recently closed). These products are exported to many countries where niches are available for vegetable tannin extract and naturally derived adhesives.
For every 1 t of bark harvested from the trees about 5 t of timber is available. The timber is mainly traded locally. Some of the wood is converted to charcoal, part of which is exported, especially to Europe, but no production or export data are available. South Africa uses 160,000–200,000 t/year of air-dried wood in the production of dissolving pulp and exports about 1.1 million t/year of air-dried chips for pulp and paper production to Japan.
Properties
The bark of black wattle contains 30–40% high-quality tannin on dry weight basis. The tannin belongs to the group of condensed proanthocyanidins, and is a complex mixture of some 40 components, mostly polymers of (+)-catechin, (–)-robinetinidin and (+)-gallocatechin. The tannin quickly penetrates the hide, and gives a firm and durable light-coloured leather, unlike other proanthocyanidin tanning materials (e.g. mangrove extracts) which give a reddish colour. It does not precipitate in acid solution, resulting in better quality leather. It is especially suited for the manufacture of sole leather for shoes.
The tannin content varies with bark thickness, age of the tree and average annual rainfall, and decreases from the base of the trunk upwards, the bark of the branches having a low tannin content. Black wattle extract contains 60–65% tannin. Extracts, usually called ‘mimosa extract’, are commercially available in several forms, each giving different qualities to leather. Usually the plant extract is mixed with synthetic tannins for use in the leather industry.
The heartwood is pale brown with reddish markings; it is indistinctly demarcated from the pale straw-coloured sapwood. The grain often interlocked, texture moderately fine. The density of the wood ranges from 550–850 kg/m3, depending on site conditions. The wood is moderately hard to hard, durable, and fairly tough and strong. It has an energy value of about 19,700 kJ/kg and ash content of about 1.5%. The energy value of charcoal is about 32,000 kJ/kg. The density and pulp yield of black wattle make it a very attractive alternative to Eucalyptus globulus Labill.
Adulterations and substitutes
Other tanning agents, such as chromium salts and synthetic tannins (syntans, resin tannages and aldehyde tannages), are nowadays mostly used for tanning leather. Although some are highly toxic and polluting, they are considered as having a more specific activity and being more predictable and controllable in the tanning process. Sometimes chrome tanning or tanning with synthetic tannins is combined with vegetable tanning, e.g. re-tannage of chrome-tanned leather to develop special characteristics, or the use of chrome tannins for shoe upper leather and vegetable tannins for the sole.
Description
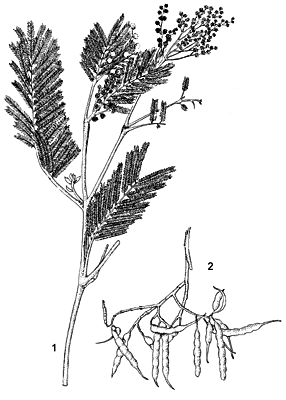
- Small to medium-sized evergreen tree up to 30 m tall; trunk straight, up to 50 cm in diameter; bark brownish-black, fissured, but in younger stems grey-brown and smooth; crown spreading, rounded at maturity; twigs unarmed, angled, grey, densely hairy when young.
- Leaves alternate, bipinnately compound, 8–15 cm long, with 8–20 pairs of pinnae 2–5 cm long; rachis with glands at base of each pair of pinnae on upper surface; leaflets very numerous, 20–70 pairs crowded on each pinna, narrowly oblong and small, 1.5–4 mm × 0.5–0.8 mm, blunt, with dense soft hairs, dark olive green.
- Inflorescence a globose head 5–8 mm in diameter, arranged in axillary racemes or panicles, head up to 50-flowered.
- Flowers bisexual, 5-merous, pale creamy-yellow, very sweet-scented; calyx lobes c. 1 mm long; corolla lobes c. 2 mm long, pointed at the apices; stamens 30–45, filaments up to 2.5 mm long; ovary superior, 1-celled, style long and slender.
- Fruit a narrowly oblong or linear, flat pod, (3–)5–10(–15) cm × 0.5–1 cm, constricted between the seeds, pubescent, dark brown to blackish when ripe, dehiscent along one suture, 3–14-seeded.
- Seeds ovoid, 3–5 mm × 2–3.5 mm, smooth and black, with a small yellowish-white aril.
Other botanical information
There has been considerable confusion about Acacia mearnsii and some closely allied species: Acacia decurrens Willd. (green wattle) and Acacia dealbata Link (silver wattle). These species have long been considered as conspecific with Acacia mearnsii, although usually distinguished as varieties. They are now usually thought to represent distinct species. The name Acacia mollissima has often been used erroneously for Acacia mearnsii. In fact, Acacia mollissima Willd. is a synonym for Acacia pubescens (Vent.) W.T.Aiton. The name Acacia decurrens is still commonly used for Acacia mearnsii, which makes literature on these species very confusing. Acacia mearnsii can be crossed with Acacia decurrens, Acacia dealbata, Acacia baileyana F.Muell. and Acacia irrorata Sieber ex Spreng. Hybrids have no real advantages over the parent species and are often partly sterile.
Anatomy
Wood-anatomical description (IAWA hardwood codes):
- Growth rings: 2: growth ring boundaries indistinct or absent.
- Vessels: 5: wood diffuse-porous; 13: simple perforation plates; 22: intervessel pits alternate; 23?: shape of alternate pits polygonal; 25: intervessel pits small (4–7 µm); (26: intervessel pits medium (7–10 µm)); 29: vestured pits; 30: vessel-ray pits with distinct borders; similar to intervessel pits in size and shape throughout the ray cell; (36: helical thickenings in vessel elements present); (37: helical thickenings throughout body of vessel element); 41: mean tangential diameter of vessel lumina 50–100 µm; 42: mean tangential diameter of vessel lumina 100–200 µm; 47: 5–20 vessels per square millimetre; 58: gums and other deposits in heartwood vessels.
- Tracheids and fibres: 61: fibres with simple to minutely bordered pits; 66: non-septate fibres present; 70: fibres very thick-walled.
- Axial parenchyma: (78: axial parenchyma scanty paratracheal); 79: axial parenchyma vasicentric; (83: axial parenchyma confluent); 91: two cells per parenchyma strand; 92: four (3–4) cells per parenchyma strand.
- Rays: 97: ray width 1–3 cells; 104: all ray cells procumbent; 115: 4–12 rays per mm.
- Mineral inclusions: 136: prismatic crystals present; 142: prismatic crystals in chambered axial parenchyma cells.
Growth and development
Seeds stay viable for many years, both in storage and in the ground. Seeds in the ground start to germinate after a forest fire or land cultivation. Seedlings are susceptible to fire. On germination, the radicle grows downwards to form a taproot of about 1 m. Fast-growing lateral roots develop from the taproot just below the soil surface. Nodules formed by N-fixing bacteria usually appear on the lateral roots.
Black wattle is a light-demanding species with rapid early stem growth, reaching up to 3 m/year. Some trees start to flower when only about 2 years old. If the growing point is not damaged black wattle produces a fairly straight stem, sometimes almost circular but usually more or less elliptical in cross-section. Plantation grown trees have an average taper of 2.5 cm in 3 m, single trees show more taper. In commercial plantations in South Africa, trees at a rotation of 10 years are rarely shorter than 10 m and on good sites attain 27–30 m, with diameters at breast height seldom exceeding 30 cm. Crown shape in plantation trees usually assumes an inverted pear-shape with age, and is generally globose at maturity. Branches die progressively from below, due to shading and competition, and at maturity the crown occupies about one fifth of the stem length. The coppicing ability of black wattle is generally poor.
The flowers are predominantly insect (bee) pollinated. Copious fruiting normally occurs after 5–6 years; fruits mature in 12–14 months. The lifespan is generally 15–20 years, but some seed orchards in South Africa are nearly 30 years old and still producing seed.
In some areas, such as Hawaii and parts of South Africa, black wattle has become a noxious weed due to its aggressive colonization of denuded areas and riparian zones.
Ecology
In its natural area of distribution, black wattle occurs in the understorey of tall open forests, in fringes of closed forests or in dense thickets on recolonized lands. Its range is from sea-level to 900 m altitude, but it mainly occurs from sea-level to about 200 m in areas with a warm subhumid to humid climate. The mean maximum temperature of the hottest month is 21–28°C, the mean minimum of the coolest month 1–7°C, with up to 40 frost days. Annual rainfall varies from (450–)625–1000(–1600) mm. Black wattle is sensitive to severe drought and to frosts of –4°C or lower. It is also very sensitive to snow damage, either snapping or bending.
In tropical countries plantations occur under wetter conditions than in the natural area of distribution. These plantations are found in highlands (1500–2500 m) with a mean annual temperature of 12–20°C, mean minimum temperature of the coolest month 2–8°C, mean maximum temperature of the hottest month 18–24°C and mean annual precipitation of 700–2000 mm. In South Africa black wattle is cultivated at 300–1000 m altitude where the climate is intermediate between that of the tree’s native range and tropical conditions.
Black wattle tolerates a variety of soils, but grows best in moist, well-drained, relatively deep and light-textured soils with pH 5–6.5. It does not grow on poorly-drained, calcareous or very infertile sites.
Propagation and planting
Black wattle is usually propagated by seed, either sown directly in the field, or in a nursery. Seed weight is low; 1 kg contains 50,000–80,000 seeds. Germination is rapid if seeds have been pretreated with very hot water (90°C). Sometimes mechanical scarification is used. Seeds retain their viability for several (to over 50) years. Vegetative propagation is not very successful but rooted cuttings, bud grafts and tissue culture have been successful in South Africa. Normally no inoculation with Rhizobium is needed.
Standard cultural practices can be used to raise seedlings in the nursery. Plantation sites should be well prepared by ploughing or soil ripping for establishment, but for re-establishment pitting is sufficient land preparation.
When seedlings are used in Zimbabwe, the initial stocking is about 2500 stems/ha, or a spacing of 2.7 m × 1.5 m. This is reduced to 2000 stems/ha when trees are 4 m tall and to 1500–1700 stems/ha when they reach 7 m. Naturally regenerated stands are initially thinned into lines and then thinned to the same spacing as planted stands. When seedlings are used in South Africa, the initial stocking is about 2200 stems/ha, which is reduced to 1600 stems/ha in one or two thinning operations. The direct seeding method uses 3–5 kg/ha of seed, sown in drill lines. The large numbers of plants that grow are then rigorously thinned and initial management is more intense.
Normally black wattle is not mixed with other species because its rapid growth hinders their development.
Management
During the first year plantations should be weeded. To maintain vigorous growth, thinning should start as early as 14 months after planting, and should be repeated at least once. The degree of thinning depends on the management objectives; severe thinning favours stem diameter growth and bark production; denser stands are needed for good timber production.
Corrective pruning is necessary if the growing point of young plants has been damaged (e.g. by browsing) and multiple leaders have developed. Care should be taken to control erosion, especially when plantations are burnt (e.g. to promote regeneration). If properly managed, black wattle may help to enrich soil nitrogen as a result of rhizobial nitrogen fixation, and rehabilitate degraded lands.
Diseases and pests
The most common disease of black wattle is black butt. The disease was first described early in the 20th century as part of the disease complex known as gummosis. Black butt may kill the tree but it also affects the yield and quality of the bark. Associated pathogens include Phytophthora spp. and Botryosphaeria dothidea. In Zimbabwe, black butt occurs mainly below 1250 m altitude. A serious disease of black wattle in South Africa is wattle wilt caused by Ceratocystis albofundus. In the humid tropics, most damage occurs from fungal attacks of Armillaria, Corticium, Fomes and Phytophthora spp. under conditions with more than 3000 mm annual precipitation.
In its native range, black wattle is not cultivated because of serious damage by indigenous insects including the fireblight beetle Acacicola orphana (synonym: Pyrgoides orphana); sometimes severe damage may occur in Brazil, too. In most tropical countries, disease and pest attacks are generally not serious, although attacks by various insects, including defoliators (e.g. wattle bagworm, Chaliopsis junodi), stem-borers (e.g. Platypus solidus), and caterpillars (e.g. wattle looper caterpillar, Achaea lienardi) may occur. In Zimbabwe and South Africa, the brown wattle mirid or froghopper Lygidolon laevigatum is the major pest affecting mainly young plantations. It attacks the growing point of the leading shoot and upper branches causing stunted growth and a witches-broom appearance. Black wattle is also attacked by cutworms (Agrotis spp.) and whitegrubs (Scarabaeid larvae, e.g. Lepidiota mashona).
Harvesting
Plantations for tannin bark are usually harvested after (7–)8–10(–12) years, when trees are more than 18 m tall and have a diameter of at least 15 cm. The bark is harvested by ripping it at several points near the base of the stem with a hatchet or short iron bar flattened at the end; the loosened strips of bark are pulled from the stem. After stripping, the bark is cut to bundle length; in Zimbabwe this is about 1.2 m. Stripping is easiest during periods of active growth. In Zimbabwe, timber is windrowed for later recovery against pole orders, but much of it cannot be sold and is burnt during subsequent land preparation.
Yield
In South Africa typical yields of fertilized plantations are 15–25 m3/ha per year of wood and 1.5–2 t/ha of dry bark. In tropical regions, and with good management, yields range between 25 m3/ha and 35 m3/ha per year of wood and from 0.9–2 t/ha of dry bark. At the best sites 60–65% of the yield consists of first grade bark from stems of at least 15 cm diameter, on poorer sites the proportion is only 40–50%.
Handling after harvest
The harvested bark is either transported immediately or dried locally first. In South Africa all bark is delivered fresh to the mill. In Zimbabwe most bark is processed fresh, but bark harvested after the milling season is dried for processing during the next season. When dried bark is used, drying should be done in partial shade; the inner bark darkens if exposed to direct sunlight. The bark discolours if it is re-wetted after drying. To obtain good bark quality, kiln drying is practised sometimes. In Indonesia trials have been done on portable charcoal-burning drying kilns in which the bark can be completely dried in about 60 hours. During drying the bark curls inwards; these ‘sticks’ are bundled for transport. During processing, the bark may either be extracted or prepared for marketing as dry bark. Fresh bark is preferred for extraction. Dry bark is graded according to thickness, maturity, lightness of colour, absence of corkiness and freedom from mould. It is marketed as chopped bark, ground bark or sometimes as dust, in pressed bales or in bags.
Genetic resources
It is thought that the seed used for black wattle plantations outside Australia originated from a limited part of the natural range. Germplasm collections exist at the CSIRO Division of Forest Research, Canberra, Australia and at the Institute for Commercial Forestry Research (ICFR), formerly the Wattle Research Institute (WRI), Pietermaritzburg, South Africa. Some provenance testing has been done, e.g. by the ICFR and in China, but further studies are needed.
Breeding
Major breeding objectives are enhanced vigour, better bark quality and stem form, and resistance to pests and diseases. The emphasis in the breeding programme in South Africa has shifted from improved bark yield and quality to improved timber yield and quality with acceptable bark yield and quality.
Prospects
Due to substitution of plastics for leather and the subsequent decline in the importance of tannin since the 1960s, black wattle cultivation has decreased in importance. However, due to the growing importance of renewable resources versus synthetic products made from oil-tar, leather and skins tanned with vegetable tannins are likely to regain some of their former economic importance. Black wattle is a potential substitute for synthetic tannins, which are widely used in the tanning industry although toxic for the workers and damaging the environment. More recently the demand for black wattle timber in South Africa, and for export, has led to the conversion of some eucalypt plantations to black wattle. Woodlots of black wattle are being planted by rural farmers in South Africa for fuelwood and as a source of building material. Black wattle is important because of its multipurpose functions and its adaptability to a wide range of ecological conditions, including degraded sites. Special consideration should be given to using black wattle for soil rehabilitation in local land use systems.
Major references
- Booth, T.H. & Jovanovic, T., 1988. Climatology of Acacia mearnsii. 1. Characteristics of natural sites and exotic plantations. New Forests 2: 17–30.
- Dunlop, R. & Hagedorn, S., 1998. Final report on two Australian Acacia mearnsii (black wattle) provenance trials established in KwaZulu-Natal and south eastern Mpumalanga. ICFR Bulletin 7/98. Institute for Commercial Forestry Research, Pietermaritzburg, South Africa.
- Herbert, M.A., 1993. Site requirements of exotic hardwood species. ICFR Bulletin 2/93. Institute for Commercial Forestry Research, Pietermaritzburg, South Africa.
- Hillis, W.E., 1997. Wood properties and uses. In: Brown, A.G. & Ho Chin Ko (Editors). Black wattle and its utilisation. RIRDC Publication No 97/72. p. 89.
- Luyt, I.E., Mullin, L.J. & Gwaze, D.P., 1987. Black wattle (Acacia mearnsii) in Zimbabwe. In: Turnbull, J.W. (Editor). Australian acacias in developing countries. Proceedings of an international workshop, held at Gympy, Queensland, Australia., 4–7 August 1986. ACIAR Proceedings No 16. Australian Centre for International Agricultural Research, Canberra, Australia. pp. 128–131.
- Nakashima, Y., Ge, J.J. & Sakai, K., 1996. Preparation and characteristics of low-density polyurethane foams derived from the barks of Acacia mearnsii and Cryptomeria japonica. Mokuzai Gakkaishi - Journal of the Japan Wood Research Society 42: 1105–1112.
- Ohara, S., 1994. Chemistry and utilization of condensed tannins from tree bark. Japan Agricultural Research Quarterly (JARQ) 28: 70–78.
- Roux, J., Kemp, G.H.J. & Wingfield, M.J., 1995. Diseases of black wattle in South Africa: a review. South African Forestry Journal 174: 35–40.
- Santana, M.A.E., Baumann, M.G.D. & Conner, A.H., 1996. Phenol-formaldehyde plywood adhesive resins prepared with liquified bark of black wattle (Acacia mearnsii). Journal of Wood Chemistry and Technology 16: 1–19.
- Sherry, S.P., 1971. The black wattle (Acacia mearnsii De Wild.). University of Natal Press, Pietermaritzburg, South Africa. 402 pp.
Other references
- Berenschot, L.M., Filius, B.M. & Hardjosoediro, S., 1988. Factors determining the occurrence of the agroforestry system with Acacia mearnsii in Central Java. Agroforestry Systems 6(2): 119–135.
- Coster, C., 1939. De betekenis van de cultures van Acacia decurrens in Nederlandsch Indië. Tectona 32: 368–388.
- Ferguson, J.H.A., 1948. Opbrengsttafels voor Acacia decurrens Willd. var. mollis Lindl., bewerkt naar nagelaten tabellen van Dr. H.E. Wolff von Wülfing, 1945. Tectona 38: 283–290.
- Hannah, B.C., Fergus, B.J. & Jones, R.N., 1977. Kraft pulping and bleaching studies on young exotic hardwood species. Appita 30: 483–487.
- InsideWood, undated. [Internet] http://insidewood.lib.ncsu.edu/search/. May 2007.
- Martin, P., 1994. A laymans guide to the pulp and papermaking industry in South Africa. Mondi. Group Training Unit, South Africa. p. 25.
- Nixon, K.M., 1992. The wattle genetics and tree breeding saga. ICFR internal document. Unpublished.
- Roux, J., 2000. Phytophthora root disease of Acacia mearnsii. Tree Pathology Co-operative Programme Leaflet. University of Pretoria.
- Roux, D.G., Ferreira, D., Botha, J.J. & Garbutt, D.C.F., 1976. Heartwood extracts of the black wattle (Acacia mearnsii) as a possible source of resorcinol. Applied Polymer Symposium No 28: 1365–1376.
- Salim, A.S., Simons, A.J., Waruhiu, A., Orwa, C. & Anyango, C., 2002. Agroforestree Database. [Internet] World Agroforestry Centre (ICRAF), Nairobi, Kenya. http://www.worldagroforestry.org/ Sites/TreeDBS/ aft.asp. 2002.
- Turnbull, J.W., 1986. Acacia mearnsii. In: Turnbull, J.W. (Editor). Multipurpose Australian trees and shrubs; lesser-known species for fuelwood and agroforestry. Australian Centre for International Agricultural Research, Canberra, Australia. pp. 164–167.
- Wiersum, K.F., 1991. Acacia mearnsii. In: Lemmens, R.H.M.J. & Wulijarni-Soetjipto, N. (Editors). Plant Resources of South-East Asia No 3. Dye and tannin-producing plants. Pudoc, Wageningen, Netherlands. pp. 41–45.
- Wingfield, M.J., De Beer, C., Visser, C. & Wingfield, B.D., 1996. A new Ceratocystis species defined using morphological and ribosomal DNA sequence comparisons. Systematic and Applied Microbiology 19: 191–202.
Sources of illustration
- Lemmens, R.H.M.J. & Wulijarni-Soetjipto, N. (Editors), 1991. Plant Resources of South-East Asia No 3. Dye and tannin-producing plants. Pudoc Scientific Publishers, Wageningen, Netherlands. 196 pp.
Author(s)
- R.W. Dunlop, Institute for Commercial Forestry Research, P.O. Box 100281, Scottsville 3209, South Africa
Correct citation of this article
Dunlop, R.W., 2005. Acacia mearnsii De Wild. In: Jansen, P.C.M. & Cardon, D. (Editors). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. Accessed 1 June 2025.
- See the Prota4U database.