Abelmoschus esculentus (PROTA)
Introduction |
| General importance | |
| Geographic coverage Africa | |
| Geographic coverage World | |
| Fruit | |
| Vegetable | |
| Vegetable oil | |
| Stimulant | |
| Medicinal | |
| Timber | |
| Ornamental | |
| Forage / feed | |
| Fibre | |
| Food security | |
Abelmoschus esculentus (L.) Moench
- Protologue: Methodus: 617 (1794).
- Family: Malvaceae
- Chromosome number: 2n = (66–)130(–144)
Synonyms
- Hibiscus esculentus L. (1753).
Vernacular names
- Common okra, okra, okro, lady’s finger (En).
- Gombo commun, gombo, gumbo (Fr).
- Quiabeiro (Po).
- Mbamia, mbinda (Sw).
Origin and geographic distribution
The genus Abelmoschus originated in South-East Asia. Abelmoschus esculentus, however, is a cultigen of uncertain origin. It is widespread in tropical, subtropical and warm temperate regions, but is particularly popular in West Africa, India, the Philippines, Thailand and Brazil. Abelmoschus esculentus has been reported from the whole of tropical Africa, whereas West African okra (Abelmoschus caillei (A.Chev.) Stevels) is restricted to the humid and perhumid climates of Africa.
Uses
Young immature fruits are an important vegetable, consumed cooked or fried. In West Africa they are usually boiled in water to make slimy soups and sauces. The fruits can be conserved by drying, whole or sliced, or by pickling. Before selling, the dried product is usually ground to powder. Young leaves are commonly used as spinach. The leaves are sometimes used as cattle feed.
Okra mucilage is suitable for medicinal and industrial applications. It has been used as a plasma replacement or blood volume expander. Leaves are sometimes used as a basis for poultices, as an emollient, sudorific or antiscorbutic and to treat dysuria. Okra mucilage has been added as size to glaze paper and is used in confectionery. The bark fibre has been locally used for fishlines and game traps. It is suitable for spinnning into rope and for paper and cardboard manufacture. Roasted okra seeds are used in some areas as a substitute for coffee.
Production and international trade
World production of okra (both species) as fresh fruit-vegetable is estimated at 6 million t/year. Common okra makes up 95% of this amount. It is only in West and Central Africa (accounting for about 10% of world production) that common okra and West African okra are both used. They share the market roughly fifty-fifty.
Properties
The composition of okra fruits per 100 g edible portion (81% of the product as purchased, ends trimmed) is: water 88.6 g (85.7–90.2), energy 144 kJ (36 kcal), protein 2.1 g (1.1–3.0), fat 0.2 g, carbohydrate 8.2 g, fibre 1.7 g, Ca 84 mg (55–142), P 90 mg, Fe 1.2 mg (1.1–1.5), ß-carotene 185 µg (180–190), thiamin 0.04 mg, riboflavin 0.08 mg, niacin 0.6 mg, ascorbic acid 47 mg (20–126). The composition of okra leaves per 100 g edible portion is: water 81.5 g (75.3–92.4), energy 235 kJ (56 kcal), protein 4.4 g (2.8–5.6), fat 0.6 g, carbohydrate 11.3 g, fibre 2.1 g, Ca 532 mg (258–635), P 70 mg, Fe 0.7 mg, ß-carotene 385 µg, thiamin 0.25 mg, riboflavin 2.8 mg, niacin 0.2 mg, ascorbic acid 59 mg (9–75) (Leung, W.-T.W., Busson, F. & Jardin, C., 1968). Compared to other fleshy fruit-vegetables (tomato, eggplant), okra is particularly rich in Ca and ascorbic acid.
Carbohydrates are mainly present in the form of mucilage. That of young fruits consists of long-chain molecules with a molecular weight of about 170,000 made up of sugar units and amino acids. The main components are galactose (25%), rhamnose (22%), galacturonic acid (27%) and amino acids (11%). The mucilage is highly soluble in water. Its solution in water has an intrinsic viscosity value of about 30.
Okra seeds contain about 20% protein (similar in amino acid composition to soya bean protein) and 20% oil (similar in fatty acid composition to cotton-seed oil). The bark fibre is easy to extract. It is white to yellow in colour, strong but rather coarse. Tests conducted in China suggest that an alcohol extract of Abelmoschus leaves can eliminate oxygen free radicals, alleviate renal tubular-interstitial diseases, improve renal function and reduce proteinuria.
Description
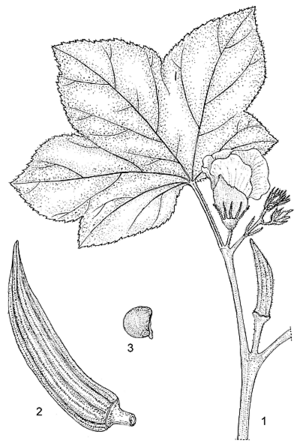
- Stout, annual, erect herb up to 4 m tall, more or less strongly branched; stem terete, with scattered, stiff hairs, glabrescent, often red-blotched; branches erect to curved downwards.
- Leaves arranged spirally, simple, variable in shape and size; stipules filiform, up to 2 cm long, often split to the base, covered with stiff hairs; petiole up to 50 cm long, often red-tinged, with a line of soft, simple hairs on the upper side, otherwise with scattered, stiff hairs and glabrescent; blade transversally elliptical to orbicular in outline, up to 50 cm broad, length of midrib up to 35 cm, mostly 3-, 5- or 7-palmatilobed to palmatipartite, cordate at base, 5–9-veined, segments triangular, ovate, elliptical, obovate, oblong, spatulate or lanceolate, acuminate, serrate to crenate, sometimes entire or angular, veins on both sides with scattered, stiff hairs, glabrescent.
- Flowers axillary, solitary or racemose by reduction or abortion of the upper leaves; pedicel up to 3 cm long in flower, up to 7 cm in fruit, with scattered, stiff hairs, glabrescent; epicalyx segments 7–15, free, linear to lanceolate, 5–25 mm × 0.5–3 mm, acute to acuminate, caducous at flowering or soon after, covered with stiff hairs; calyx spathaceous, 2–6 cm long, 5-toothed apically, usually splitting on one side at the expansion of the corolla, adnate to and caducous with the corolla and staminal column, strigose to sericeous; petals 5, free, obovate to orbicular, 3–7 cm long, base fleshy, apex obtuse to retuse, glabrous, yellow, often turning pink after anthesis, with a dark purple centre; stamens united into a staminal column up to 2.5 cm long, white, glabrous; ovary superior, tomentose, often with some stiff hairs on the costae as well, 5–10 style arms 3–5 mm long, stigmas dark purple, with simple hairs.
- Fruit an erect, cylindrical to pyramidal capsule 5–25 cm × 1–5 cm, acuminate, terete to 5–10-angled, concave between the costae, gradually losing its original indumentum, when young varying in colour from purple-red and reddish-green to dark green, and from pale green to yellow, completely or partially loculicidal or not opening at all, up to 100-seeded.
- Seeds globose to ovoid, 3–6 mm in diameter, with minute warts in concentric rows, rarely with long red hairs on the seed coat.
- Seedling with epigeal germination.
Other botanical information
Abelmoschus esculentus (usually 2n = 130) is probably an amphidiploid (allotetraploid), derived from Abelmoschus tuberculatus Pal & H.B.Singh (2n = 58), a wild species from India, and a species with 2n = 72 chromosomes (possibly Abelmoschus ficulneus (L.) Wight & Arn. ex Wight).
Another edible okra species, Abelmoschus caillei (A.Chev.) Stevels, occurs in the humid parts of West and Central Africa. There are strong indications that also Abelmoschus caillei is an amphidiploid with Abelmoschus esculentus being one of the parental species.
There are no apparent differences in use between the common and West African okra, which is why they are often lumped together. Morphologically Abelmoschus caillei differs in several respects from Abelmoschus esculentus, but the epicalyx offers the best discriminating characteristic: the width of the epicalyx segments is 0.5–3 mm in Abelmoschus esculentus and 4–13 mm in Abelmoschus caillei. The two okra species can be quite reliably (but not with absolute certainty) recognized on the basis of fruit form. Fruits of Abelmoschus esculentus are cylindrical to pyramidal, whereas fruits of Abelmoschus caillei are ovoid. Literature references on common okra have to be interpreted with care because they may include information related to Abelmoschus caillei.
There are many cultivars of common okra. Some of the better known are ‘Clemson Spineless’, ‘Indiana’, ‘Emerald’ (United States) and ‘Pusa Sawani’ (India), which have been in use for about 30 years.
Growth and development
Under the conditions of southern Côte d’Ivoire (5°N), local and introduced cultivars flower within 45–80 days after sowing in the dry season (sowing in October: days shortening) and within 55–105 days after sowing in the rainy season (sowing in March: days lengthening). Crop duration rarely exceeds 6 months.
Flower opening and pollination take place in the early morning. Although basically a self-pollinated crop, considerable cross-pollination by insects may take place. For vegetable use the fruits are picked about one week after anthesis. The regular removal of young fruits permits sustained vegetative growth and flowering, prolonging the productive period. In a seed crop, it takes about one month from anthesis to fruit maturity. In this case, vegetative growth stops soon after anthesis, all assimilates being diverted to the reproductive plant parts.
Ecology
Abelmoschus esculentus needs temperatures above 20°C for normal growth and development. Germination percentage and speed of emergence are optimal at 30–35°C. Flower initiation and flowering are delayed with increasing temperatures (positive correlation between temperature and number of vegetative nodes). Abelmoschus esculentus is a short-day plant, but its wide geographical distribution (up to latitudes of 35–40°) indicates that cultivars differ markedly in sensitivity. Flower initiation and flowering are hardly affected by daylength in popular subtropical cultivars such as ‘Clemson Spineless’ and ‘Pusa Sawani’. Most tropical cultivars show quantitative short-day responses, but qualitative responses also occur. The shortest critical daylength reported is 12 hours 30 minutes. This explains why flowering of local cultivars of common okra is only quantitatively affected by daylength in the coastal areas of the Gulf of Guinea (5°N). However, more inland at higher latitudes (10°N) one can occasionally observe very tall non-flowering plants of common okra due to a qualitative response.
Common okra tolerates a wide variety of soils but prefers well-drained sandy loams, with pH 6–7, and a high content of organic matter.
Propagation and planting
Most farmers harvest seed from their own local cultivar or rather heterogeneous landrace. The easiest way to keep the seed is to leave it in the pods. Seed weight varies from 30–80 g/1000 seeds. To soften the hard seed coat, the seed is often soaked in water or chemicals prior to sowing. The seed is usually dibbled directly in the field (1–3 seeds per hole). Optimum plant densities are in the range of 50,000–150,000 plants/ha. Emergence is within one week. When the plants are about 10 cm tall, they are thinned to one plant per hole.
Germination and initial growth are improved greatly by cultural practices that lower soil temperature, e.g. mulching, watering before the hottest part of the day, and sowing on ridge sides least exposed to direct sunlight.
Management
Commercial okra growers usually practise sole cropping, and prefer the early, homogeneous, introduced cultivars. In traditional agriculture, farmers grow their okra landraces in home gardens or in fields with other food crops. In West and Central Africa the landraces often consist of a mixture of Abelmoschus esculentus and Abelmoschus caillei, the former being predominant in dry climates, the latter in humid climates.
The uptake of minerals is rather high. Indicative figures for total nutrient uptake per ha of a crop with a fruit yield of about 10 t/ha are 100 kg N, 10 kg P, 60 kg K, 80 kg Ca and 40 kg Mg.
Under humid tropical conditions a full-grown crop consumes about 8 mm of water per day.
Some farmers practise ratoon cropping. A ratoon crop flowers soon after cutting, but usually results in poor quality fruit with a high percentage of bent fruits.
Diseases and pests
The most serious fungal diseases of okra in Africa are damping-off (Macrophomina phaseolina, Pythium aphanidermatum, Rhizoctonia solani), vascular wilt (Fusarium oxysporum), Cercospora blight (Cercospora abelmoschi, Cercospora malayensis) and powdery mildew (Erysiphe cichoracearum, Oidium abelmoschi).
Okra mosaic virus (OkMV), transmitted by flea beetles (Podagrica), is widespread in Africa but damage is much less important than that caused by okra leaf curl disease (OLCV), transmitted by whitefly (Bemisia tabaci). Whitefly is also the vector of yellow vein mosaic virus (BYVMV), a major cause of crop failure in Asia. These viruses can only be controlled through control of the vectors. Nematodes of the genus Meloidogyne constitute a major problem. Damage by nematodes is avoided by crop rotation (e.g. with cereals) and by large applications of organic manure.
Important pests are fruit and stem borers (Earias spp. and Heliothis spp., Pectinophora gossypiella), flea beetles (Podagrica spp.) and jassids (Empoasca spp.). Chemical control is hazardous because crop harvesting is frequent. Common okra is in general more seriously affected by diseases and pests than West African okra.
Harvesting
The earliest types of common okra are ready for first harvest at 7 weeks after sowing. Fruits should be harvested when 7–8 days old. Earlier picking depresses yields because of low fruit weight, but delayed picking depresses marketable yields because over-aged fruits become fibrous. Okra fields are, therefore, harvested at intervals of 2–3 days. The minimum frequency is once a week but then fruits of all sizes have to be picked. Although such a low frequency reduces yield, the very small fruits can fetch a higher price, being of prime quality. For seed production, the whole crop can be harvested once-over. Intensive contact with the slightly hairy fruits and plants may lead to skin irritation.
Yield
A vegetable yield of 10 t/ha can be considered a good harvest, but yields of over 40 t/ha can be realized under optimal conditions. Yields are usually low (2–4 t/ha ) as a result of non-intensive growing methods. Seed yields are in the range of 500–1000 kg/ha.
Handling after harvest
Fresh okra can be transported quite easily in bulk and kept for a few days without much loss of quality. Dried okra is an important product in West Africa. Although usually sliced transversally, longitudinal slicing has been observed in Benue State, Nigeria. Such slices dry well at the edges but start fermenting from the middle, creating a unique flavour. Some countries have a small canning and freezing industry.
Okra mucilage can be obtained by grinding plant material, removing waxes and fat with ether and alcohol, suspending the purified material in water, filtering and concentrating the filtrate.
Genetic resources
Local landraces in Africa are not at great risk of genetic erosion at present. Only commercial growers tend to switch to commercial cultivars of common okra, whereas local landraces of both okra species are ubiquitous in subsistence farming.
Germplasm base collections are maintained by the Southern Regional Plant Introduction Station (Griffin, Georgia, United States), NHR (National Horticultural Research Institute, Ibadan, Nigeria), IRD (Institut de Recherche pour le Développement, Montpellier, France), CNRA (Centre National de Recherches Agronomiques, Bouaké, Côte d’Ivoire), NBPGR (National Bureau for Plant Genetic Resources, New Delhi, India) and IPB (Institute of Plant Breeding, Los Baños, the Philippines).
Breeding
In Africa selection and breeding of common okra have only been carried out to a limited scale by the commercial sector. Technisem Seed Company in Senegal distributes improved African cultivars, e.g. ‘Volta’ suitable for the hot and cool season, and the F1 hybrid ‘Lima’ with high tolerance to virus diseases and suitable for export. African farmers have selected an enormous diversity of forms which fit within a great variety of cropping systems. Some of these are available from local seed houses. International breeding work has been oriented towards intensive cultivation with high production in a short period (early maturity, compact plants with short internodes, high-density planting) and wide adaptation (photoperiod insensitivity, resistance to pests and diseases). Crossing between promising parents combined with pedigree selection or backcrossing remains the most common breeding procedure. Several attractive American and Indian cultivars have found their way to commercial growers throughout the tropics and subtropics, but there is still plenty of scope for cultivar improvement in Africa for the commercial sector (where good alternatives for the introduced cultivars are needed with better adaptation to local conditions) as well as for the traditional sector (where hardy, robust, long-lived types are required). Nevertheless, isozyme analysis has shown a rather low level of genetic diversity in cultivated okra in spite of much phenotypic variability. There is little information on improvement using biotechnology apart from in-vitro DNA extraction and plant regeneration from various explants and callus tissue.
The characteristics of both okra species open up new opportunities for recombination. They cross readily in both directions and crosses result in vigorous hybrids; these, however, show a marked reduction in fertility. Nevertheless, seed is formed by interspecific hybrids under conditions of open pollination, probably due to backcrossing with fertile pollen of one of the parental species. ‘Parbhani Kranti’ was bred in this way in India, with YVMV resistance/tolerance derived from Abelmoschus caillei.
Although occurring together in farmers’ fields in West Africa, the genetic integrity of the two okra species is largely assured because chances are very small that the unproductive F1 hybrids will be selected as seed sources for the next crop.
Prospects
Okra will remain a welcome, productive fruit-vegetable. The relatively recent discovery that West African okra is different from common okra offers new possibilities in an old crop. Okra improvement will also greatly benefit from a better understanding of the phylogeny and species relations within the genus Abelmoschus.
Major references
- Charrier, A., 1984. Genetic resources of Abelmoschus (okra). International Board for Plant Genetic Resources (IBPGR), Rome, Italy. 61 pp.
- Düzyaman, E., 1997. Okra: botany and horticulture. Horticultural Reviews 21: 41–72.
- Hamon, S., 1988. Organisation évolutive du genre Abelmoschus (gombo). Co-adaptation et évolution de deux espèces de gombo cultivées en Afrique de l’Ouest, A. esculentus et A. caillei. Travaux et Documents Microédités (TDM) No 46. ORSTOM, Paris, France. 191 pp.
- Hamon, S. & Charrier, A., 1997. Les gombos. In: Charrier, A., Jacquot, M., Hamon, S. & Nicolas, D. (Editors). L’amélioration des plantes tropicales. Centre de coopération internationale en recherche agronomique pour le développement (CIRAD) & Institut français de recherche scientifique pour le développement en coopération (ORSTOM), Montpellier, France. pp. 313–333.
- Hamon, S. & Hamon, P., 1991. Future prospects of the genetic integrity of two species of okra (Abelmoschus esculentus and A. caillei) cultivated in West Africa. Euphytica 58: 101–111.
- IBPGR, 1991. Report of an international workshop on okra genetic resources, held at the National Bureau for Plant Genetic Resources (NBPGR), New Delhi, India, 8–12 October 1990. International Crop Network Series 5. International Board for Plant Genetic Resources (IBPGR), Rome, Italy. 133 pp.
- Schippers, R.R., 2000. African indigenous vegetables. An overview of the cultivated species. Natural Resources Institute/ACP-EU Technical Centre for Agricultural and Rural Cooperation, Chatham, United Kingdom. 214 pp.
- Siemonsma, J.S., 1982. West African okra - morphological and cytogenetical indications for the existence of a natural amphidiploid of Abelmoschus esculentus (L.) Moench and A. manihot (L.) Medikus. Euphytica 31: 241–252.
- Siemonsma, J.S., 1982. La culture du gombo (Abelmoschus spp.), légume-fruit tropical (avec référence spéciale à la Côte d’Ivoire). PhD thesis Wageningen Agricultural University, Wageningen, Netherlands. 297 pp.
- Stevels, J.M.C., 1988. Une nouvelle combinaison dans Abelmoschus Medik. (Malvaceae), un gombo d’Afrique de l’Ouest et Centrale. Bulletin du Muséum National d’Histoire Naturelle, Paris, séries 4, 10, section. B, Adansonia 2: 137–144.
Other references
- Burkill, H.M., 1997. The useful plants of West Tropical Africa. 2nd Edition. Volume 4, Families M–R. Royal Botanic Gardens, Kew, Richmond, United Kingdom. 969 pp.
- Chevalier, A., 1940. L’origine, la culture et les usages de cinq Hibiscus de la section Abelmoschus. Revue de Botanique Appliquée 20: 319–328, 402–419.
- Hamon, S. & van Sloten, D.H., 1995. Okra. In: Smartt, J. & Simmonds, N.W. (Editors). Evolution of crop plants. 2nd Edition. Longman, London, United Kingdom. pp. 350–357.
- Leung, W.-T.W., Busson, F. & Jardin, C., 1968. Food composition table for use in Africa. FAO, Rome, Italy. 306 pp.
- Markose, B.L. & Peter, K.V., 1990. Okra. Review of research on vegetables and tuber crops. Technical Bulletin 16. Kerala Agricultural University Press, Mannuthy, Kerala, India. 109 pp.
- Martin, F.W. & Ruberté, R.M., 1978. Vegetables for the hot humid tropics. Part 2. Okra, Abelmoschus esculentus. Mayagüez Institute of Tropical Agriculture, Puerto Rico, United States. 22 pp.
- Siemonsma, J.S., 1991. Abelmoschus: a taxonomical and cytogenetical overview. In: IBPGR. Report of an international workshop on okra genetic resources, held at the National Bureau for Plant Genetic Resources (NBPGR), New Delhi, India, 8–12 October 1990. International Crop Network Series 5. International Board for Plant Genetic Resources (IBPGR), Rome, Italy. pp. 52–68.
- Stevels, J.M.C., 1990. Légumes traditionnels du Cameroun: une étude agrobotanique. Wageningen Agricultural University Papers 90–1. Wageningen Agricultural University, Wageningen, Netherlands. 262 pp.
- Tomoda, M., Shimada, K., Saito, Y. & Sugi, M., 1980. Plant mucilages. 26. Isolation and structural features of a mucilage, ‘Okra-mucilage F’, from the immature fruits of Abelmoschus esculentus. Chemical and Pharmaceutical Bulletin 28: 2933–2940.
- van Borssum-Waalkes, J., 1966. Malaysian Malvaceae revised. Blumea 14: 1–251.
Sources of illustration
- Stevels, J.M.C., 1990. Légumes traditionnels du Cameroun: une étude agrobotanique. Wageningen Agricultural University Papers 90–1. Wageningen Agricultural University, Wageningen, Netherlands. 262 pp.
Author(s)
- J.S. Siemonsma, PROTA Network Office Europe, Wageningen University, P.O. Box 341, 6700 AH Wageningen, Netherlands
- C. Kouamé, Centre National de Recherches Agronomiques (CNRA), 01 B.P. 1740, Abidjan 01, Côte d’Ivoire
Correct citation of this article
Siemonsma, J.S. & Kouamé, C., 2004. Abelmoschus esculentus (L.) Moench. [Internet] Record from PROTA4U. Grubben, G.J.H. & Denton, O.A. (Editors). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. <http://www.prota4u.org/search.asp>.
Accessed 12 December 2024.