Difference between revisions of "Phaseolus coccineus (PROTA)"
| (One intermediate revision by the same user not shown) | |||
| Line 12: | Line 12: | ||
| Food security= 2 | | Food security= 2 | ||
}} | }} | ||
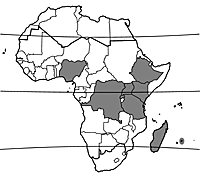
| − | [[File:Map Phaseolus coccineus.gif|thumb|]] | + | [[File:Map Phaseolus coccineus.gif|thumb|distribution in Africa (planted)]] |
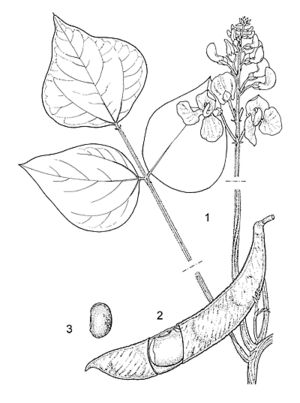
| − | [[File:Linedrawing Phaseolus coccineus.gif|thumb|]] | + | [[File:Linedrawing Phaseolus coccineus.gif|thumb|1, part of flowering branch; 2, fruit; 3, seed. Source: PROSEA]] |
| − | [[File:Phaseolus coccineus | + | [[File:Phaseolus coccineus.jpg|thumb|plant habit]] |
| − | [[File:Phaseolus coccineus 6280.jpg|thumb|]] | + | [[File:Phaseolus coccineus 6280.jpg|thumb|flowering plant]] |
| − | [[File:Phaseolus coccineus 7991.jpg|thumb|]] | + | [[File:Phaseolus coccineus 7991.jpg|thumb|red-flowering plant]] |
| − | [[File:Phaseolus coccineus.jpg|thumb|]] | + | [[File:Phaseolus coccineus 3238.jpg|thumb|detail of fruiting plant]] |
| − | [[File:Phaseolus coccineusWAG9000509.jpg|thumb|]] | + | [[File:Phaseolus coccineusWAG9000509.jpg|thumb|different cultivars]] |
| − | [[File:Phaseolus coccineusWAG9000510.jpg|thumb|]] | + | [[File:Phaseolus coccineusWAG9000510.jpg|thumb|cv. ‘Painted Lady’]] |
| − | + | ||
<big>''[[Phaseolus coccineus]]'' L.</big> | <big>''[[Phaseolus coccineus]]'' L.</big> | ||
__NOTOC__ | __NOTOC__ | ||
Latest revision as of 17:23, 28 August 2015
Introduction |
| General importance | |
| Geographic coverage Africa | |
| Geographic coverage World | |
| Cereal / pulse | |
| Vegetable | |
| Carbohydrate / starch | |
| Medicinal | |
| Ornamental | |
| Forage / feed | |
| Food security | |
- Protologue: Sp. pl. 2: 724 (1753).
- Family: Papilionaceae (Leguminosae - Papilionoideae, Fabaceae)
- Chromosome number: 2n = 22
Vernacular names
- Scarlet runner bean, runner bean, multiflora bean, case knife bean (En).
- Haricot d’Espagne (Fr).
- Feijão da Espanha, feijão escarlata, feijão trepador (Po).
Origin and geographic distribution
Scarlet runner bean occurs wild from Mexico to Panama. It was probably domesticated in Mexico. Archaeological evidence indicates that scarlet runner bean was a domesticated crop in Mexico around 900 AD. Nowadays scarlet runner bean is cultivated in temperate countries and occasionally in highland areas of Central and South America, Africa (e.g. Ethiopia, Kenya, Uganda, South Africa) and Asia. It is probably cultivated in Madagascar and is recorded as being grown in the eastern part of tropical southern Africa, although no specific countries are mentioned.
Uses
In Central America the immature and mature seeds are consumed, elsewhere mainly the mature seeds, e.g. in Ethiopia. Preparation is predominantly by boiling. In temperate regions the immature pods are most commonly eaten, sliced and cooked, as a vegetable. In Central America the young shoots, leaves and inflorescences are sometimes used (boiled or boiled and fried) as a vegetable while the tuberous roots are consumed boiled or chewed as candy. A root decoction is taken against malaria or applied to swollen eyes. In Central America scarlet runner bean is grazed by livestock and dried into hay. It is grown as an ornamental.
Production and international trade
Accurate production statistics for scarlet runner bean are not available. Production is almost exclusively for local use. Commercial production of the pods is done in the United Kingdom and Argentina and of the seeds of white-seeded cultivars in South Africa. In Kenya scarlet runner bean is mainly grown by smallholders.
Properties
Per 100 g edible portion the composition of dried scarlet runner bean seeds is: water 12.5 g, energy 1415 kJ (338 kcal), protein 20.3 g, fat 1.8 g, carbohydrate 62.0 g, fibre 4.8 g, Ca 114 mg, P 354 mg, Fe 9.0 mg, thiamin 0.50 mg, riboflavin 0.19 mg, niacin 2.3 mg and ascorbic acid 2 mg (Leung, Busson & Jardin, 1968). The seeds contain antinutritional factors such as trypsin inhibitors, and must be cooked before being eaten to break down these compounds.
Per 100 g edible portion, raw green pods (ends and sides trimmed) contain: water 91.2 g, energy 93 kJ (22 kcal), protein 1.6 g, fat 0.4 g, carbohydrate 3.2 g, fibre 2.6 g, Ca 33 mg, Mg 19 mg, P 34 mg, Fe 1.2 mg, Zn 0.2 mg, carotene 145 μg, thiamin 0.06 mg, riboflavin 0.03 mg, niacin trace and ascorbic acid 18 mg (Holland, Unwin & Buss, 1991). Many improved cultivars have substantial reduction in the fibrous vascular strands of the pod sutures (‘stringless runner beans’).
The tuberous root of scarlet runner bean is edible, but it is fibrous and may contain toxic compounds, which can be removed by soaking or peeling and by discarding the cooking water.
Coccinin, a peptide isolated from the seed of scarlet runner bean, has shown antifungal activity against a range of fungi. It also inhibited proliferation in leukaemia cell lines and reduced the activity of HIV-1 reverse transcriptase.
Description
- Perennial, climbing herb with stems up to 4(–7) m long or bushy annual herb up to 60 cm tall; taproot tuberous.
- Leaves alternate, 3-foliolate; stipules triangular; petiole (6–)8. 5–10.5(–16) cm long, rachis (1.5–)2.5–4(–5) cm long; stipels c. 5 mm long; leaflets ovate-rhombic, (5–)6.5–10.5(–12.5) cm × (3.5–)5–8.5(–12.5) cm, base cuneate or truncate, apex acute, thinly pubescent to glabrescent.
- Inflorescence an axillary or terminal raceme, many-flowered; peduncle (5–)11–16.5(–25.5) cm long; rachis (2–)10–16(–39.5) cm long.
- Flowers bisexual, papilionaceous; pedicel 0.5–1.5 cm long; calyx campanulate, glabrescent, tube c. 3 mm long, the upper 2 lobes united, the lower 3 triangular, c. 1 mm long; corolla scarlet, pink or white, standard hood-shaped, circular or broadly obovate, c. 17 mm × 17 mm, wings broadly obovate, c. 25 mm × 17 mm, keel coiled, c. 10 mm long; stamens 10, 9 fused and 1 free; ovary superior, c. 6 mm long, finely pubescent, style coiled, with collar of hairs below the stigma.
- Fruit a linear-lanceolate, straight or slightly curved pod (4.5–)9–13(–30) cm × 1.5–2.5 cm, laterally compressed, beaked, glabrescent, rough with small oblique ridges, (1–)3–5(–10)-seeded.
- Seeds ellipsoid-oblong, 13–25 mm × 6–13(–16) mm, black, white, cream or brown, often pink to purple mottled.
- Seedling with hypogeal germination; first pair of leaves simple and opposite.
Other botanical information
Phaseolus comprises about 50 species, most of them in the Americas. Phaseolus coccineus is closely related to Phaseolus dumosus Macfad. (synonym: Phaseolus polyanthus Greenman; year-bean, sometimes also called runner bean) and Phaseolus costaricensis Freytag & Debouck. Hybrids between Phaseolus coccineus and these 2 species have been obtained; natural hybridization also occurs. The 3 species can be crossed with common bean (Phaseolus vulgaris L.), with the latter as female parent, without embryo rescue, although progenies may be partially sterile. Where scarlet runner bean and common bean grow together, natural hybridization may occur. Hybridization of scarlet runner bean with tepary bean (Phaseolus acutifolius A.Gray) is also possible.
Phaseolus coccineus is a variable species, and levels of genetic variability are high, both in wild and in cultivated populations. A white-seeded type of Phaseolus coccineus is known as ‘butter bean’ in Kenya and South Africa, but this name is normally applied to Phaseolus lunatus L. In Uganda, where the crop is grown a high altitudes in Nakuru District, white-seeded cultivars are most common.
Growth and development
Scarlet runner bean seeds germinate 10–14 days after sowing. Flowering starts 40–60 days after sowing. Flowers open at sunrise and fade at sunset. Phaseolus coccineus is predominantly cross-pollinating. Harvesting of green pods starts around 3 months after sowing and can be easily sustained for 2–3 months. Mature seed can be harvested 4–5 months after sowing. Bushy cultivars produce earlier and smaller crops than climbing cultivars. In Central America scarlet runner bean is sometimes grown as a perennial: where stems die back during cooler periods, the tuberous taproot remains viable and produces new stems with returning warmth. In temperate regions scarlet runner bean is grown as an annual. Scarlet runner bean fixes atmospheric nitrogen by symbiosis with fast-growing Rhizobium bacteria.
Ecology
Scarlet runner bean is a crop for temperate climates. In the tropics it is most successful at altitudes of 1500–2000 m. In Kenya it is grown at 1900–2600 m altitude, in Ethiopia up to about 2000 m. Scarlet runner bean is more tolerant of cool conditions than other Phaseolus species, but damage occurs at temperatures below 5°C. At temperatures above 25°C fruit development is inhibited. Scarlet runner bean is extremely susceptible to drought and requires a well-distributed rainfall throughout the growing period. In Ethiopia it is successfully grown in areas with an average annual rainfall of 1500 mm. It needs a high relative humidity for seed set. Scarlet runner bean comprises short-day and day-neutral types.
Scarlet runner bean is adapted to a wide range of soils, but it prefers deep, well-drained, loamy, light- to medium-textured soils, with pH 6–7. Waterlogging is not tolerated.
Propagation and planting
Scarlet runner bean is normally propagated by seed, but the tuberous root with a piece of stem can also be used. The 1000-seed weight is 700–3000 g. The seedbed should be well prepared and weed free. Normal planting densities are 50,000–75,000 plants/ha for climbing types and double those for bushy types, requiring about 75 kg and 150 kg seed per ha, respectively. However, lower densities have also been recorded. In Mauritius scarlet runner bean is sown in rows 100 cm apart with 30 cm within the row. The sowing depth is 2.5–5 cm. In Central America scarlet runner bean is often intercropped with maize.
Management
To obtain high-quality pods, scarlet runner bean is grown on trellises, poles, fence lines or other support structures. However, labour and material requirements are high and may impede cultivation. Climbing types can yield without support if leading shoots are pinched out to induce bushy growth. Scarlet runner bean should be kept weed-free during the early growth stages and it is commonly weeded once or twice. Tillage should be shallow to avoid root damage. Supplementary irrigation is beneficial. In Ethiopia scarlet runner bean is a garden crop.
Diseases and pests
In the tropics scarlet runner bean is affected by anthracnose (Colletotrichum lindemuthianum) and Fusarium wilt (Fusarium solani f.sp. phaseoli). The seed-borne disease halo blight (Pseudomonas savastanoi pv. phaseolicola, synonym: Pseudomonas syringae pv. phaseolicola) has been isolated from scarlet runner bean in South Africa.
Harvesting
Green pods of scarlet runner bean are harvested when pod length reaches its maximum before the phase of rapid seed development. Picking is usually at 4–5 day intervals. For dry seed production, plants are pulled or cut when most pods are dry and then allowed to dry for a few days. Alternatively pods may be handpicked in several rounds because of asynchronous ripening.
Yield
Yields of green pods of 10 t/ha and of seeds of 1.5 t/ha are possible. The yield of dry mature seeds in Kenya has been estimated at 900–1100 kg/ha.
Handling after harvest
After drying, scarlet runner bean pods are threshed.
Genetic resources
In Brazil 428 accessions are maintained by EMBRAPA/CENARGEN in Brasilia. Large germplasm collections of scarlet runner bean are also maintained in the United States (USDA-ARS Western Regional Plant Introduction Station, Pullman, Washington, 478 accessions from throughout the world including Ethiopia and Kenya) and Mexico (Banco Nacional de Germoplasma Vegetal, Universidad Autónoma Chapingo, Chapingo, 311 accessions). In Africa 6 accessions are kept in South Africa (Division of Plant and Seed Control, Department of Agriculture, Pretoria) and 1 in Ethiopia (International Livestock Research Institute (ILRI), Addis Ababa).
Breeding
Breeding efforts for scarlet runner bean have been directed to improvement of culinary quality (stringlessness) and disease resistance. Selection to improve cooking quality is promising since seed proteins of scarlet runner bean are more polymorphic than those of common bean. For dry seed production, improvement of plant habit and shorter pods are appropriate objectives of selection. Cultivars with a determinate growth habit suitable for mechanical harvesting (‘Venere’ and ‘Alarico’) have been developed in Italy, by crossing Phaseolus coccineus with determinate Phaseolus vulgaris cultivars and repeated backcrossing with Phaseolus coccineus.
Moderate levels of resistance to common bacterial blight (Xanthomonas campestris pv. phaseoli), Fusarium root rot (Fusarium solani f.sp. phaseoli) and white mould (Sclerotinia sclerotiorum) have been transferred from scarlet runner bean to common bean. Scarlet runner bean is also considered as a potential source of resistance against other diseases of common bean, including anthracnose, Ascochyta blight (Phoma exigua), angular leaf spot (Phaeoisariopsis griseola), powdery mildew (Erysiphe polygoni) and rust (Uromyces appendiculatus). Considerable tolerance to bean flies (Ophiomyia spp.) has been detected in scarlet runner bean, and tolerance has been transferred into common bean. On the other hand, resistance to halo blight has been transferred from common bean to scarlet runner bean.
In vitro plant regeneration of scarlet runner bean is possible using cotyledons, through direct organogenesis as well as somatic embryogenesis via callus.
Prospects
Scarlet runner bean is a suitable pulse and vegetable crop for the humid highland tropics, although the need to provide support and the uneven maturation of the pods are serious drawbacks for commercial production. Scarlet runner bean may have some potential at higher altitudes in tropical Africa, but more information is needed on appropriate sowing and management practices. It is a potential source of resistance to diseases and pests affecting common bean.
Major references
- Campion, B., 1995. ‘Venere’ and ‘Alarico’, new scarlet runner bean (Phaseolus coccineus L.) cultivars with determinate growth habit. HortScience 30(7): 1483–1484.
- Debouck, D.G. & Smartt, J., 1995. Beans. In: Smartt, J. & Simmonds, N.W. (Editors). Evolution of crop plants. 2nd Edition. Longman, London, United Kingdom. pp. 287–294.
- Freytag, G. & Debouck, D.G., 2002. Taxonomy, distribution and ecology of the genus Phaseolus (Leguminosae - Papilionoideae) in North America, Mexico and Central America. Botanical Institute of Texas, Fort Worth, Texas, United States. 300 pp.
- Gepts, P. (Editor), 1988. Genetic resources of Phaseolus beans: their maintenance, domestication, evolution, and utilization. Kluwer Academic Publishers, Dordrecht, Netherlands. 613 pp.
- Kay, D.E., 1979. Food legumes. Crops and Product Digest No 3. Tropical Products Institute, London, United Kingdom. 435 pp.
- Singh, S.P., 2001. Broadening the genetic base of common bean cultivars: a review. Crop Science 41(6): 1659–1675.
- Smartt, J., 1989. Phaseolus coccineus L. In: van der Maesen, L.J.G. & Somaatmadja, S. (Editors). Plant Resources of South-East Asia No 1. Pulses. Pudoc, Wageningen, Netherlands. pp. 56–57.
- Suttie, J.M., 1969. The butter bean (Phaseolus coccineus L.) in Kenya. East African Agricultural and Forestry Journal 35: 211–212.
- Westphal, E., 1974. Pulses in Ethiopia, their taxonomy and agricultural significance. Agricultural Research Reports 815. Centre for Agricultural Publishing and Documentation, Wageningen, Netherlands. 263 pp.
- Webster, B.D., Ross, R.M. & Sigourney, M.C., 1980. A morphological study of the development of reproductive structures of Phaseolus coccineus Lam. Journal of the American Society for Horticultural Science 105(6): 828–833.
Other references
- Duke, J.A., 1981. Handbook of legumes of world economic importance. Plenum Press, New York, United States, and London, United Kingdom. 345 pp.
- du Puy, D.J., Labat, J.N., Rabevohitra, R., Villiers, J.-F., Bosser, J. & Moat, J., 2002. The Leguminosae of Madagascar. Royal Botanic Gardens, Kew, Richmond, United Kingdom. 750 pp.
- Escalante, A.M., Coello, G., Eguiarte, L.E. & Piñero, D., 1994. Genetic structure and mating systems in wild and cultivated populations of Phaseolus coccineus and P. vulgaris (Fabaceae). American Journal of Botany 81(9): 1096–1103.
- FAO, 1989. Utilization of tropical foods: tropical beans. Compendium on technological and nutritional aspects of processing and utilization of tropical foods, both animal and plant, for purposes of training and field reference. FAO Food and Nutrition paper 47/4. FAO, Rome, Italy. 74 pp.
- Fourie, D., 1998. Characterization of halo blight races on dry beans in South Africa. Plant Disease 82(3): 307–310.
- Hidalgo, R. & Beebe, S., 1997. Phaseolus beans. In: Fuccillo, D., Sears, L. & Stapleton, P. (Editors). Biodiversity in trust: conservation and use of plant genetic resources in CGIAR centres. Cambridge University Press, Cambridge, United Kingdom. pp. 139–155.
- Holland, B., Unwin, I.D. & Buss, D.H., 1991. Vegetables, herbs and spices. The fifth supplement to McCance & Widdowson’s The Composition of Foods. 4th Edition. Royal Society of Chemistry, Cambridge, United Kingdom. 163 pp.
- Kaplan, L. & Lynch, T.F., 1999. Phaseolus (Fabaceae) in archaeology: AMS radiocarbon dates and their significance for pre-Colombian agriculture. Economic Botany 53(3): 261–272.
- Knudsen, K. (Editor), 2000. Directorio de colecciones de germoplasma en América Latina y el Caribe. International Plant Genetic Resources Institute (IPGRI), Rome, Italy. 369 pp.
- Leung, W.-T.W., Busson, F. & Jardin, C., 1968. Food composition table for use in Africa. FAO, Rome, Italy. 306 pp.
- Liebenberg, A.J., 1995. Dry bean research in South Africa with special emphasis on the institutes of the Agricultural Research Council. Report of the Bean Improvement Cooperative No 38. pp. 17–18.
- Mahuku, G.S., Jara, C.E., Cajiao, C. & Beebe, S., 2002. Sources of resistance to Colletotrichum lindemuthianum in the secondary gene pool of Phaseolus vulgaris and in crosses of primary and secondary gene pools. Plant Disease 86(12): 1383–1387.
- Mahuku, G.S., Jara, C.E., Cajiao, C. & Beebe, S., 2002. Sources of resistance to angular leaf spot (Phaeioisariopsis griseola) in common bean core collection, wild Phaseolus vulgaris and secondary gene pool. Euphytica 130(3): 303–313.
- Nagl, W., Ignacimuthu, S. & Becker, J., 1997. Genetic engineering and regeneration of Phaseolus and Vigna. State of the art and new attempts. Journal of Plant Physiology 150(6): 625–644.
- Ngai, P.H.K. & Ng, T.B., 2004. Coccinin, an antifungal peptide with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from large scarlet runner beans. Peptides 25(12): 2063–2068.
- Schmit, V. & Baudoin, J.P., 1992. Screening for resistance to Ascochyta blight in populations of Phaseolus coccineus L. and P. polyanthus Greenman. Field Crops Research 30: 155–165.
- Smartt, J., 1976. Tropical pulses. Longman, London, United Kingdom. 348 pp.
- Summerfield, R.J. & Roberts, E.H. (Editors), 1985. Grain legume crops. Collins, London, United Kingdom. 859 pp.
- Thulin, M., 1989. Fabaceae (Leguminosae). In: Hedberg, I. & Edwards, S. (Editors). Flora of Ethiopia. Volume 3. Pittosporaceae to Araliaceae. The National Herbarium, Addis Ababa University, Addis Ababa, Ethiopia and Department of Systematic Botany, Uppsala University, Uppsala, Sweden. pp. 49–251.
- Yu, Z.H., Stall, R.E. & Vallejos, C.E., 1998. Detection of genes for resistance to common bacterial blight of beans. Crop Science 38(5): 1290–1296.
Sources of illustration
- Smartt, J., 1989. Phaseolus coccineus L. In: van der Maesen, L.J.G. & Somaatmadja, S. (Editors). Plant Resources of South-East Asia No 1. Pulses. Pudoc, Wageningen, Netherlands. pp. 56–57.
Author(s)
- M. Brink, PROTA Network Office Europe, Wageningen University, P.O. Box 341, 6700 AH Wageningen, Netherlands
Correct citation of this article
Brink, M., 2006. Phaseolus coccineus L. In: Brink, M. & Belay, G. (Editors). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. Accessed 18 December 2024.
- See the Prota4U database.