Difference between revisions of "Gomphocarpus fruticosus (PROTA)"
| (6 intermediate revisions by the same user not shown) | |||
| Line 16: | Line 16: | ||
{{DISPLAYTITLE:''Gomphocarpus fruticosus'' (PROTA)}} | {{DISPLAYTITLE:''Gomphocarpus fruticosus'' (PROTA)}} | ||
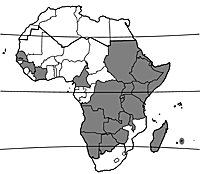
[[File:Gomphocarpus fruticosus PROTA map.png|thumb|distribution in Africa (wild)]] | [[File:Gomphocarpus fruticosus PROTA map.png|thumb|distribution in Africa (wild)]] | ||
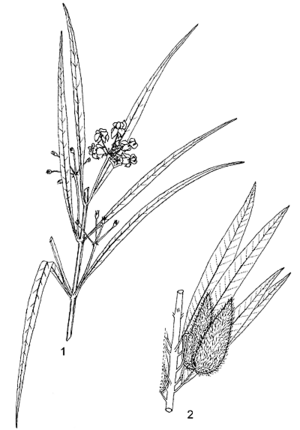
| + | [[File:Linedrawing Gomphocarpus fruticosus.gif|thumb|1, flowering twig; 2, fruiting twig. | ||
| + | Redrawn and adapted by Iskak Syamsudin]] | ||
| + | [[File:Gomphocarpus fruticosus Zimbabweflora.jpg|thumb|flowers ([http://www.zimbabweflora.co.zw/speciesdata/image-display.php?species_id=145690&image_id=3 Flora of Zimbabwe])]] | ||
:Protologue: Hort. kew. (ed. 2) 2: 80 (1811). | :Protologue: Hort. kew. (ed. 2) 2: 80 (1811). | ||
| Line 68: | Line 71: | ||
== Description == | == Description == | ||
| − | Shrubby perennial, much branched from the base, up to 1.5(–2.5) m tall, all parts with latex, branches erect, densely hairy when young, woody at base, with taproot. Leaves opposite, simple and entire; petiole 1–10 mm long; blade linear to narrowly linear-elliptical, (2.5–)4–12 cm × (0.2–)0.3–0.8(–1.3) cm, base cuneate, apex attenuate, mucronate, yellowish-green, sparsely to densely hairy with soft white hairs on veins. Inflorescences an extra-axillary nodding umbel, 4–7(–12)-flowered; peduncle 1.5–3(–4) cm long; bracts filiform, deciduous. Flowers bisexual, regular, 5-merous; pedicel 1–2.5 cm long; sepals lanceolate or triangular, 2–5 mm long, attenuate; corolla reflexed, white, yellowish or pink, lobes ovate, 5–8 mm × 3–5 mm, acute, margins ciliate; corona lobes attached 1–1.5 mm above base of staminal column, laterally compressed, 2–4 mm × 1.5–3 mm, as tall as the column, upper margins entire, proximal margins a pair of falcate teeth c. 1–1.5 mm long, pointing back along the upper margins of the lobe or curved down into the cavity; anther wings 1.5–2 mm long; ovary superior, carpels 2, free, stigma head flat. Fruit a pair of upright follicles, each follicle ovoid, 4–7 cm × 1.5–2.5 cm, tapering gradually or abruptly into a long beak, strongly or weakly inflated, balloon-like, papery, pale green, sometimes tinged reddish, short-hairy, with or without soft spiny processes, many-seeded. Seeds ovate with one convex and one concave face, 3.5–5 mm × c. 2 mm, warted, brownish grey, coma 2.5–3 cm long. | + | *Shrubby perennial, much branched from the base, up to 1.5(–2.5) m tall, all parts with latex, branches erect, densely hairy when young, woody at base, with taproot. |
| + | |||
| + | *Leaves opposite, simple and entire; petiole 1–10 mm long; blade linear to narrowly linear-elliptical, (2.5–)4–12 cm × (0.2–)0.3–0.8(–1.3) cm, base cuneate, apex attenuate, mucronate, yellowish-green, sparsely to densely hairy with soft white hairs on veins. | ||
| + | |||
| + | *Inflorescences an extra-axillary nodding umbel, 4–7(–12)-flowered; peduncle 1.5–3(–4) cm long; bracts filiform, deciduous. | ||
| + | |||
| + | *Flowers bisexual, regular, 5-merous; pedicel 1–2.5 cm long; sepals lanceolate or triangular, 2–5 mm long, attenuate; corolla reflexed, white, yellowish or pink, lobes ovate, 5–8 mm × 3–5 mm, acute, margins ciliate; corona lobes attached 1–1.5 mm above base of staminal column, laterally compressed, 2–4 mm × 1.5–3 mm, as tall as the column, upper margins entire, proximal margins a pair of falcate teeth c. 1–1.5 mm long, pointing back along the upper margins of the lobe or curved down into the cavity; anther wings 1.5–2 mm long; ovary superior, carpels 2, free, stigma head flat. | ||
| + | |||
| + | *Fruit a pair of upright follicles, each follicle ovoid, 4–7 cm × 1.5–2.5 cm, tapering gradually or abruptly into a long beak, strongly or weakly inflated, balloon-like, papery, pale green, sometimes tinged reddish, short-hairy, with or without soft spiny processes, many-seeded. | ||
| + | |||
| + | *Seeds ovate with one convex and one concave face, 3.5–5 mm × c. 2 mm, warted, brownish grey, coma 2.5–3 cm long. | ||
== Other botanical information == | == Other botanical information == | ||
| Line 76: | Line 89: | ||
Several other ''Gomphocarpus'' species in tropical Africa are used medicinally. | Several other ''Gomphocarpus'' species in tropical Africa are used medicinally. | ||
| − | === ''Gomphocarpus cancellatus'' | + | === ''Gomphocarpus cancellatus'' and ''G. filiformis'' === |
| − | + | ||
''[[Gomphocarpus cancellatus]]'' (Burm.f.) Bruyns and ''[[Gomphocarpus filiformis]]'' (E.Mey.) D.Dietr. occur in Namibia and South Africa. Dried and powdered roots or aerial parts are used as a snuff to treat influenza and colds. | ''[[Gomphocarpus cancellatus]]'' (Burm.f.) Bruyns and ''[[Gomphocarpus filiformis]]'' (E.Mey.) D.Dietr. occur in Namibia and South Africa. Dried and powdered roots or aerial parts are used as a snuff to treat influenza and colds. | ||
| Line 83: | Line 95: | ||
''[[Gomphocarpus glaucophyllus]]'' Schltr. occurs from Uganda south to South Africa. In Zimbabwe a root infusion is drunk to treat asthma. A root decoction is given to babies to stop vomiting. | ''[[Gomphocarpus glaucophyllus]]'' Schltr. occurs from Uganda south to South Africa. In Zimbabwe a root infusion is drunk to treat asthma. A root decoction is given to babies to stop vomiting. | ||
| − | === ''Gomphocarpus | + | === ''Gomphocarpus purpurascens'' === |
| − | ''[[Gomphocarpus | + | ''[[Gomphocarpus purpurascens]]'' A.Rich. is an endemic species of Ethiopia. The fresh or dry root bark is pounded with water and drunk to cure fever. |
=== ''Gomphocarpus solstitialis'' === | === ''Gomphocarpus solstitialis'' === | ||
| Line 92: | Line 104: | ||
''[[Gomphocarpus stenophyllus]]'' Oliv. occurs in the semi-arid regions of southern Ethiopia, Kenya and Tanzania. In Kenya a root decoction is drunk to treat diarrhoea. | ''[[Gomphocarpus stenophyllus]]'' Oliv. occurs in the semi-arid regions of southern Ethiopia, Kenya and Tanzania. In Kenya a root decoction is drunk to treat diarrhoea. | ||
| − | === ''Gomphocarpus tomentosus | + | === ''Gomphocarpus tomentosus'' === |
| − | ''[[Gomphocarpus tomentosus'' Burch. occurs throughout southern Africa. In Namibia the powder of leaves and stems is used as a snuff to treat colds with nasal discharge. A decoction of the whole plant is used as a genital wash to treat venereal sores. Powder of roasted leaves is applied to wounds and venereal sores. In Botswana dried leaves are taken as an emetic; powdered roots macerated in water are taken as an emetic after poisoning. In South Africa the Tsonga people drink a root decoction to treat intestinal worms. | + | ''[[Gomphocarpus tomentosus]]'' Burch. occurs throughout southern Africa. In Namibia the powder of leaves and stems is used as a snuff to treat colds with nasal discharge. A decoction of the whole plant is used as a genital wash to treat venereal sores. Powder of roasted leaves is applied to wounds and venereal sores. In Botswana dried leaves are taken as an emetic; powdered roots macerated in water are taken as an emetic after poisoning. In South Africa the Tsonga people drink a root decoction to treat intestinal worms. |
== Growth and development == | == Growth and development == | ||
Latest revision as of 22:43, 24 October 2014
Introduction |
| General importance | |
| Geographic coverage Africa | |
| Geographic coverage World | |
| Fruit | |
| Vegetable | |
| Medicinal | |
| Ornamental | |
| Forage / feed | |
| Fibre | |
| Food security | |
Gomphocarpus fruticosus (L.) W.T.Aiton

- Protologue: Hort. kew. (ed. 2) 2: 80 (1811).
- Family: Asclepiadaceae (APG: Apocynaceae)
- Chromosome number: 2n = 22
Synonyms
- Asclepias fruticosa L. (1753),
- Asclepias decipiens N.E.Br. (1908).
Vernacular names
- Narrow-leafed cotton plant, cotton bush, swan plant, bristle-fruited silkweed, mobydick (En).
- Faux cotonnier, fanor, petite ouate, la ouate, ouatier marron (Fr).
Origin and geographic distribution
Gomphocarpus fruticosus occurs wild throughout East and southern Africa, South Africa, as well as in Yemen, Oman and Saudi Arabia. In several other African countries, including Senegal, Guinea, Côte d’Ivoire, Cameroon, Sudan, Madagascar and Mauritius, it is naturalized. It is furthermore naturalized and rather common in northern Africa, southern Europe and eastern Australia; in Australia it is considered a noxious weed. In other countries in the tropics and subtropics it has probably escaped from cultivation more recently, and is sometimes naturalized.
Uses
Throughout eastern and southern Africa as well as Madagascar a leaf or root infusion or root and leaf powder in water is taken as an emetic. A root or plant decoction is taken to treat liver troubles, malaria and abdominal pains. Throughout southern Africa an infusion of the leaves, roots and fruit is taken orally to treat diarrhoea. In Uganda a water extract from the roots is taken to treat backache. In Namibia root tea is drunk daily to treat diabetes. In Botswana the Bayei people drink a root decoction to treat gonorrhoea. In Zimbabwe root powder is eaten to stop vomiting of bile. In South Africa a root infusion or decoction is taken to treat general body pain, diabetes and infertility. In Madagascar a root and leaf decoction is taken to treat asthma, nerve pain and as a diuretic.
In Ethiopia crushed fresh or dried leaves are applied to sores. In Namibia leaf tea is drunk and the body is rubbed with tea and leaves to treat skin cancer. In Botswana fresh powdered leaves are soaked in water and the liquid is drunk to induce vomiting in case of hepatitis. In South Africa leaf powder is used as snuff for headache as a sedative and to treat tuberculosis; it produces prolonged sneezing. In southern Africa and the Indian Ocean Islands a leaf maceration or infusion is drunk to treat stomach-ache and diarrhoea of children, and to stimulate contractions during childbirth. A leaf or whole plant infusion is also drunk to treat asthma, bronchitis, cardiac palpitations and gas formation.
In Uganda and Madagascar the latex applied to teeth to treat toothache and a decoction of the seeds is taken as a cough medicine. In Kenya, Uganda and Tanzania the latex is applied to sores, boils and wounds, on the forehead to treat headache and as ear drops to treat otitis, as a sedative. In Namibia latex is applied to warts.
In the Indian Ocean Islands a plant or seed decoction is taken 2–3 times daily against cardiac problems, as a tonic. Depending on the dose a plant decoction is considered a cardiac tonic, emetic or toxic. In Madagascar swellings are treated by massage with a plant decoction. To treat lumbago the plant is dried, ground and mixed with honey to a paste for application with massage.
Throughout East Africa a decoction of the whole plant is given to cattle with colic. In Ethiopia a fresh leaf maceration is given to cattle with anthrax and blackleg. In Namibia concentrated leaf tea is given to dogs with distemper. In Madagascar a leaf and root decoction is given to cattle with excessive gas. In Zambia the bitter latex is smeared over eggs in chicken sheds to prevent snakes and dogs from eating them. Cut plants are stuffed into rodent holes to deter them. In Namibia the San people use the latex as an arrow poison ingredient.
The inner bark yields a white fibre and is used in the way as that of Gomphocarpus physocarpus E.Mey. In Somalia and southern Africa the stem fibre and floss from the fruit are used as string for snares and to make waistbands and fishing nets. The floss is also used as tinder. In southern Africa the floss is used for stuffing mattresses and pillows. In Somalia the cleaned stems are used by shepherds for driving sheep.
In Lesotho the roots are cooked and eaten as a vegetable. In Kenya the Maasai people eat the fruit. Livestock browse the plants, but it is considered poisonous if eaten in large quantities, causing respiratory problems and severe gastroenteritis. Foliage and the fruit are used in floral arrangements also dry. When working amongst Gomphocarpus fruticosus grown as a cut-plant protective clothing is suggested, as some people have an allergic reaction to the latex. In southern Africa and Madagascar the plant enters into magic rituals and is used against bewitchment.
Production and international trade
In Kenya Gomphocarpus fruticosus has been selected as ornamental cut-flower in 2001 and production has since then shown a spectacular growth rate from 13 kg at US$ 17 in 2001 to 288,707 kg at US$ 656,170 in 2006.
On the internet seeds are sold for prices ranging from US$ 38 for 50 seeds to US$ 528 for 50,000 seeds.
Properties
All parts of Gomphocarpus fruticosus are toxic, due to the presence of cardiac glycosides (cardenolides) and to a lesser extent to pregnane glycosides. From all parts the cardenolides afroside and gomphoside were isolated. From different seed and leaf fractions the following cardiac glycosides were isolated: afroside, gomphoside, uzarigenin, uscharidin, uscharin, desglucouzarin, neouzarin, frugoside, glucofrugoside, gofruside, asclepin, calactin, gomphotoxin, gomphotin and gomphacil. The leaves also contain 5,11-epoxymegastigmane glucosides, the coumarins scopoletin and scopolin and the flavonoids kaempferol, quercetin, isorhamnetin and their 3-O-β-rutinosides. Other compounds isolated are: 3’-epi-afroside, 3’-epi-afroside 3’-acetate, 19-deoxyuscharin, 3’-didehydroafroside, 3-epi-gomphoside, 3-epi-gomphoside 3’-acetate, conditurol F and several derivatives as well as coroglaucigenin and corotoxigenin glycosides. The aerial parts contain high levels of manganese.
The latex contains the papain-like protease asclepain f. The latex has proteolytic activity. The diluted and centrifuged latex is reported to contain 276 μg of protein/ml and the proteolytic activity reached 1.2 caseinolytic U/ml. This enzyme preparation was found to be stable after 2 hours at 45°C but was quickly inactivated after 5 minutes at 80°C.
An aqueous extract of the dried and powdered aerial parts showed prolonged, irregular contractions of high amplitude in Guinea pig uterine smooth muscle in vitro. The extracts have cardiotonic and antihypertensive but not decongestant or analgesic activities. Methanolic and aqueous extracts of the aerial parts demonstrated a noteworthy growth inhibitory effect against 3 cancer cell lines with IC50 values <50 μg/ml. A methanol extract of the fruits showed significant antiplasmodial activity in vitro. Water, hexane and ethanol extracts of the dried leaves, assessed for in-vitro antibacterial activity against Staphylococcus aureus, Klebsiella pneumoniae, Bacillus subtilis and Escherichia coli were found to be inactive.
Description
- Shrubby perennial, much branched from the base, up to 1.5(–2.5) m tall, all parts with latex, branches erect, densely hairy when young, woody at base, with taproot.
- Leaves opposite, simple and entire; petiole 1–10 mm long; blade linear to narrowly linear-elliptical, (2.5–)4–12 cm × (0.2–)0.3–0.8(–1.3) cm, base cuneate, apex attenuate, mucronate, yellowish-green, sparsely to densely hairy with soft white hairs on veins.
- Inflorescences an extra-axillary nodding umbel, 4–7(–12)-flowered; peduncle 1.5–3(–4) cm long; bracts filiform, deciduous.
- Flowers bisexual, regular, 5-merous; pedicel 1–2.5 cm long; sepals lanceolate or triangular, 2–5 mm long, attenuate; corolla reflexed, white, yellowish or pink, lobes ovate, 5–8 mm × 3–5 mm, acute, margins ciliate; corona lobes attached 1–1.5 mm above base of staminal column, laterally compressed, 2–4 mm × 1.5–3 mm, as tall as the column, upper margins entire, proximal margins a pair of falcate teeth c. 1–1.5 mm long, pointing back along the upper margins of the lobe or curved down into the cavity; anther wings 1.5–2 mm long; ovary superior, carpels 2, free, stigma head flat.
- Fruit a pair of upright follicles, each follicle ovoid, 4–7 cm × 1.5–2.5 cm, tapering gradually or abruptly into a long beak, strongly or weakly inflated, balloon-like, papery, pale green, sometimes tinged reddish, short-hairy, with or without soft spiny processes, many-seeded.
- Seeds ovate with one convex and one concave face, 3.5–5 mm × c. 2 mm, warted, brownish grey, coma 2.5–3 cm long.
Other botanical information
Gomphocarpus comprises about 22 species in tropical Africa and Peninsular Arabia. In Gomphocarpus fruticosus 5 subspecies are distinguished, depending on the hairiness of the plant, the colour of the flower and the presence of processes and form of the fruit. It is found to hybridize with Gomphocarpus physocarpus.
Several other Gomphocarpus species in tropical Africa are used medicinally.
Gomphocarpus cancellatus and G. filiformis
Gomphocarpus cancellatus (Burm.f.) Bruyns and Gomphocarpus filiformis (E.Mey.) D.Dietr. occur in Namibia and South Africa. Dried and powdered roots or aerial parts are used as a snuff to treat influenza and colds.
Gomphocarpus glaucophyllus
Gomphocarpus glaucophyllus Schltr. occurs from Uganda south to South Africa. In Zimbabwe a root infusion is drunk to treat asthma. A root decoction is given to babies to stop vomiting.
Gomphocarpus purpurascens
Gomphocarpus purpurascens A.Rich. is an endemic species of Ethiopia. The fresh or dry root bark is pounded with water and drunk to cure fever.
Gomphocarpus solstitialis
Gomphocarpus solstitialis (A.Chev.) Bullock occurs throughout West Africa. In Togo a root decoction is drunk to treat stomach-ache.
Gomphocarpus stenophyllus
Gomphocarpus stenophyllus Oliv. occurs in the semi-arid regions of southern Ethiopia, Kenya and Tanzania. In Kenya a root decoction is drunk to treat diarrhoea.
Gomphocarpus tomentosus
Gomphocarpus tomentosus Burch. occurs throughout southern Africa. In Namibia the powder of leaves and stems is used as a snuff to treat colds with nasal discharge. A decoction of the whole plant is used as a genital wash to treat venereal sores. Powder of roasted leaves is applied to wounds and venereal sores. In Botswana dried leaves are taken as an emetic; powdered roots macerated in water are taken as an emetic after poisoning. In South Africa the Tsonga people drink a root decoction to treat intestinal worms.
Growth and development
Gomphocarpus fruticosus is a facultative cross-pollinator. When grown as cut-flower, plants require pinching to remove apical dominance and encourage lateral branching.
Ecology
Gomphocarpus fruticosus occurs in well-drained, dry sandy soils in grasslands, along road sides, railway lines and abandoned fields, frequently on river banks, in full sun or partial shade, from sea-level up to 2500 m altitude.
Propagation and planting
Gomphocarpus fruticosus is easily propagated by seeds and by lateral roots. Because of their deep taproot, they do not transplant readily. Best results are derived from raising plants from seeds or root cuttings and containing them in individual pots until they are planted.
Management
In Kenya discoloration of the green fruits and stem to brownish and purplish colours is a major problem of Gomphocarpus fruticosus plants grown as cut-flowers. Discoloration of the fruits has been found to be caused by plant nutrition (declining N, P, K, Ca, Mg content or increased Fe, Cu, Zn content), prolonged exposure to sunlight and water stress, although other factors, including fruit maturity, soil pH and cross pollination between different varieties may also play a role. Fruit hairlessness also makes the plants unmarketable.
Arthropod pests are controlled with neem (Azadirachta indica A.Juss.) based soil management, soil drenching with chemical pesticides and entomopathogenic fungi.
Diseases and pests
In Kenya Gomphocarpus fruticosus faces several major pest challenges when planted as a crop. Viral infections cause blackening of stem and fruits and stunting of plants, resulting in small unmarketable fruits. Pests include root knot nematodes and insects such as cotton stainers (Dysdercus sp.), aphids (Aphis gossypii), thrips and bollworms (Helicoverpa armigera), as well as mites (Tetranychus sp.). Larvae and adult beetles of Corynodes and Euryope (Chrysomelidae) feed on the roots. It is also a preferred food plant for caterpillars of Danaus plexippus, but they do not kill the plants.
Harvesting
Gomphocarpus fruticosus bears fruit almost all year round. As it tends to grow very fast, several harvests can be made in the course of the year.
Handling after harvest
The latex of Gomphocarpus fruticosus is corrosive, and causes skin irritation, so careful handling of the harvested parts is required.
Genetic resources
Gomphocarpus fruticosus is common and widespread, and there is no risk of genetic erosion.
Breeding
No breeding programs are known to exist for Gomphocarpus fruticosus grown as an ornamental cut-plant, although selection of high-yielding plants with optimal fruit formation is needed to improve its market value.
Prospects
Although chemical analyses have confirmed the presence of a range of cardiac glycosides as well as other compounds, not many pharmacological analyses have been effected to confirm the different traditional medicinal uses, and more research should therefore be undertaken. Because of the toxicity of the plant, the different plant parts should be used with care.
Concerning its use as an ornamental cut-plant, different trials are needed to determine the cause of fruit discoloration, optimum plant spacing and frequency of top pinching, as well as quantification of yield losses, identification of the plant viruses, pest population dynamics and management strategies. The latex should be analyzed to determine the level of protection needed when handling the plants. Since it is cross-pollinating, propagation through seeds leads to character segregation and likelihood of non-uniformity of fruits, internode length and plant height. Development of tissue culture protocols through meristem propagation would be useful for rapid multiplication of uniform, disease free and true to type progeny with uniform fruits and internode length. There is furthermore a need to develop maturity indices and design appropriate packaging for the fruiting stems to enhance its quality and consequent market value. Finally the value chain should be strengthened through enhanced linkages among the growers, researchers, microfinance institutions and marketing agents for local and export markets.
Major references
- Burkill, H.M., 1985. The useful plants of West Tropical Africa. 2nd Edition. Volume 1, Families A–D. Royal Botanic Gardens, Kew, Richmond, United Kingdom. 960 pp.
- Goyder, D.J. & Nicholas, A., 2001. A revision of Gomphocarpus R.Br. (Apocynaceae: Asclepiadeae). Kew Bulletin 56(4): 769–836.
- Komissarenko, A.N., Komissarenko, S. N. & Chernobai, V.T., 1997. Cardenolides, coumarins and flavonoids of Gomphocarpus fruticosus (L.) Ait.f. Rastitel'nye Resursy 33(1): 29–41.
- Komissarenko, N.F., Chernobai, V.T. & Komissarenko, A.N., 1995. New cardenolides from the leaves of Gomphocarpus fruticosus. Chemistry of Natural Compounds 31(6): 694–700.
- Mothana, R.A.A., Grünert, R., Bednarski, P.J. & Lindequist, U., 2009. Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine. Pharmazie 64(4): 260–268.
- Neuwinger, H.D., 2000. African traditional medicine: a dictionary of plant use and applications. Medpharm Scientific, Stuttgart, Germany. 589 pp.
- Waiganjo, M.M., Gikaara, D.N., Muriithi, A., Kihara, S., Kamau, E. & Gateri, M., 2008. The status of native ornamental plants and their potential utilization in the floriculture industry in Kenya. In: Jaenicke, H., Ganry, J., Hoeschle-Zeledon, I. & Kahane, R. (Editors). International symposium on underutilized plants for food, nutrition, income and sustainable development, Arusha, Tanzania.
- Warashina, T. & Noro, T., 1994. Steroidal glycosides and cardenolide glycosides from Asclepias fruticosa. Phytochemistry 37(1): 217–226.
Other references
- Abe, F., Mori, Y., Okabe, H. & Yamauchi, T., 1994. Steroidal constituents from the roots and stems of Asclepias fruticosa. Chemical and Pharmacological Bulletin 42: 1777–1783.
- Abe, F. & Yamauchi, T., 2000. 5,11-Epoxymegastigmanes from the leaves of Asclepias fruticosa. Chemical and Pharmacological Bulletin 48: 1908–1911.
- Adjanohoun, E.J., Aké Assi, L., Eymé, J., Gassita, J.N., Goudoté, E., Guého, J., Ip, F.S.L., Jackaria, D., Kalachand, S.K.K., Keita, A., Koudogbo, B., Landreau, D., Owadally, A.W. & Soopramanien, A., 1983. Médecine traditionelle et pharmacopée - Contribution aux études ethnobotaniques et floristiques à Maurice (Iles Maurice et Rodrigues). Agence de Coopération Culturelle et Technique, Paris, France. 216 pp.
- Baerts, M. & Lehmann, J., 2009. Gomphocarpus fruticosus. [Internet] Prelude Medicinal Plants Database. Metafro-Infosys, Royal Museum for Central Africa, Tervuren, Belgium http://www.metafro.be/prelude. September 2009.
- Boiteau, P., Boiteau, M. & Allorge-Boiteau, L., 1999. Dictionnaire des noms malgaches de végétaux. 4 Volumes + Index des noms scientifiques avec leurs équivalents malgaches. Editions Alzieu, Grenoble, France.
- Chagnon, M., 1984. Inventaire pharmacologique général des plantes médicinales Rwandaises. Journal of Ethnopharmacology 12: 239–251.
- Clarkson, C., Maharaj, V.J., Crouch, N.R., Grace, O.M., Pillay, P., Matsabisa, M.G., Bhagwandin, N., Smith, P.J. & Folb, P.I., 2004. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. Journal of Ethnopharmacology 92: 177–191.
- Gradé, J.T., Tabuti, J.R.S. & van Damme, P., 2009. Ethnoveterinary knowledge in pastoral Karamoja, Uganda. Journal of Ethnopharmacology 122: 273–293.
- Gurib-Fakim, A., Guého, J. & Bissoondoyal, M.D., 1995. Plantes médicinales de Maurice, tome 1. Editions de l’Océan Indien, Rose-Hill, Mauritius. 495 pp.
- Heneidak, S., Grayer, R.J., Kite, G.C. & Simmonds, M.S-J., 2006. Flavonoid glycosides from Egyptian species of the tribe Asclepiadeae (Apocynaceae, subfamily Asclepiadoideae). Biochemical Systematics and Ecology 34(7): 575–584.
- McGaw, L.J., Jäger, A.K. & van Staden, J., 2000. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. Journal of Ethnopharmacology 72: 247–263.
- SEPASAL, 2009. Gomphocarpus. [Internet] Survey of Economic Plants for Arid and Semi-Arid Lands (SEPASAL) database. Royal Botanic Gardens, Kew, Richmond, United Kingdom. http://www.kew.org/ ceb/sepasal/. September 2009.
- Sewram, V., Raynor, M.W., Raidoo, D.M. & Mulholland, D.A., 1998. Coupling SFE to uterotonic bioassay: an on-line approach to analysing medicinal plants. Journal of Pharmaceutical and Biomedical Analysis 18(3): 305–318.
- Sokolova, S.M., Lovkova, M.Y. & Buzuk, G.N., 2007. Microelements and cardiac glycosides from medicinal plants. Doklady Akademii Nauk 413(4): 571–573.
- Trejo, S.A., Lopez, L.M.I., Caffini, N.O., Natalucci, C.L., Canals, F. & Aviles, F.X., 2009. Sequencing and characterization of asclepain f: the first cysteine peptidase cDNA cloned and expressed from Asclepias fruticosa latex. Planta 230(2): 319–328.
- van Wyk, B.-E., 2008. A review of Khoi-San and Cape Dutch medical ethnobotany. Journal of Ethnopharmacology 119: 331–341.
- van Wyk, B.E., van Heerden, F. & van Oudtshoorn, B., 2002. Poisonous plants of South Africa. Briza Publications, Pretoria, South Africa. 288 pp.
- Wondimu, T., Asfaw, Z. & Kelbessa, E., 2007. Ethnobotanical study of medicinal plants around ‘Dheeraa’ town, Arsi Zone, Ethiopia. Journal of Ethnopharmacology 112: 152–161.
Sources of illustration
- Berhaut, J., 1971. Flore illustrée du Sénégal. Dicotylédones. Volume 1. Acanthacées à Avicenniacées. Gouvernement du Sénégal, Ministère du Développement Rural et de l’Hydraulique, Direction des Eaux et Forêts, Dakar, Senegal. 626 pp.
- Leeuwenberg, A.J.M. & Rudjiman, 2005. Apocynacées. In: Autry, J.C., Bosser, J. & Ferguson, I.K. (Editors). Flore des Mascareignes. Famille 121–126. Institut de Recherche Scientifique pour le Développement, Paris, France, Mauritius Sugar Industry Research Institute, Mauritius & Royal Botanic Gardens, Kew, Richmond, United Kingdom. 31 pp.
Author(s)
- A. Gurib-Fakim, Faculty of Science, University of Mauritius, Réduit, Mauritius
Correct citation of this article
Gurib-Fakim, A., 2011. Gomphocarpus fruticosus (L.) W.T.Aiton. In: Schmelzer, G.H. & Gurib-Fakim, A. (Editors). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. Accessed 23 December 2024.
- See the Prota4U database.